Human amniotic membrane-derived mesenchymal stem cell pretreatment method and application thereof
A technology of stem cells and human amniotic membrane, applied in the fields of medicine and hematology, can solve the problems of tumorigenicity, unsafety, high immunogenicity, etc., to enhance the role of SDF-1/CXCR4 axis, improve the success rate and The effect of curative effect and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
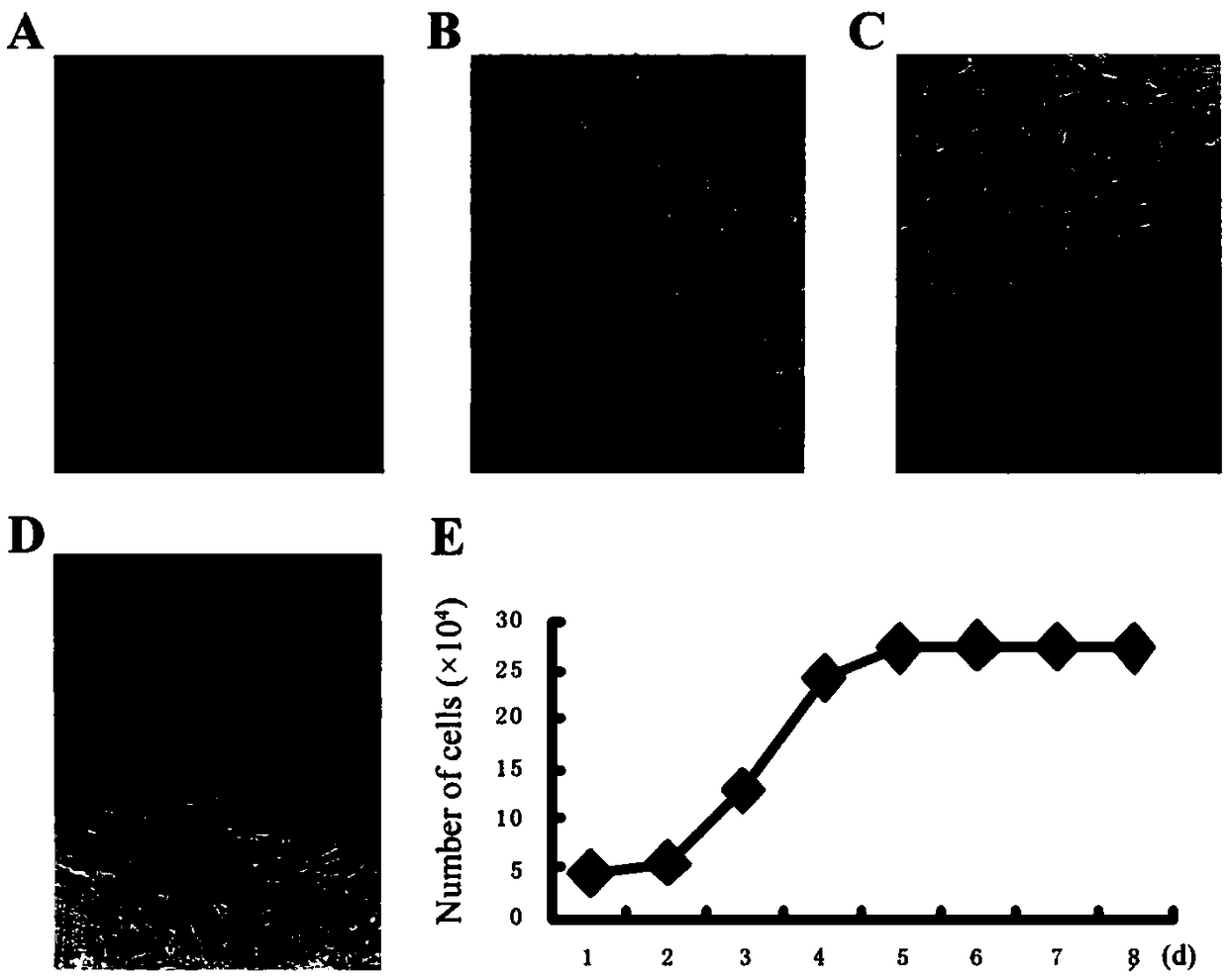
[0066] The separation culture, purification and expansion of embodiment 1AMSC
[0067] (1) Material collection: Under sterile conditions, take the placenta of a healthy full-term cesarean section fetus (serological reactions such as hepatitis, HIV, and syphilis are all negative, with the informed consent of the parturient), and bluntly separate about 10 cm of the amniotic membrane on the fetal side of the placenta. ×10cm, rinse repeatedly to remove blood clots.
[0068] (2) Cutting: Cut the amniotic membrane into about 1mm 3 small pieces, in the shape of mince.
[0069] (3) Inoculation: Use a straw to suck up some small pieces of tissue, put them into a culture bottle, and inoculate the small pieces of tissue on the bottom of the culture bottle. The distance between the small pieces is about 0.5cm to 1cm. 2 The culture bottle can be inoculated with 45-60 pieces.
[0070](4) Culture: After the inoculation, add about 3ml of complete DMEM / F12 medium, gently turn the culture bo...
Embodiment 2
[0076] Isolation, cultivation, purification and expansion of AEC
[0077] (1) The method of amniotic membrane sampling is the same as before.
[0078] (2) Use tweezers to pick up several pieces of amniotic tissue into a centrifuge tube, add 5ml of 0.125% trypsin, and place it in a 37°C incubator for digestion for 3 times, each time for about 40 minutes. During the digestion period, shake the centrifuge tube once every 20 minutes. . Digestion was terminated with serum. Collect the digested liquid for 3 times, filter through a 200-mesh sieve, centrifuge at 1000r / min for 5min.
[0079] (3) Resuspend the cells with complete DMEM / F12 medium, with 6×10 5 / cm 2 Inoculated at 75cm 2 In the culture flask, add complete DMEM / F12 medium to 15ml.
[0080] (4) Change the medium for the first time after 48 hours, and then change the medium every 2 days. After about 5 to 7 days, the adherent cells have reached 80-90% fusion with each other. Pour off the old culture medium, wash it twice...
Embodiment 3 Embodiment 1
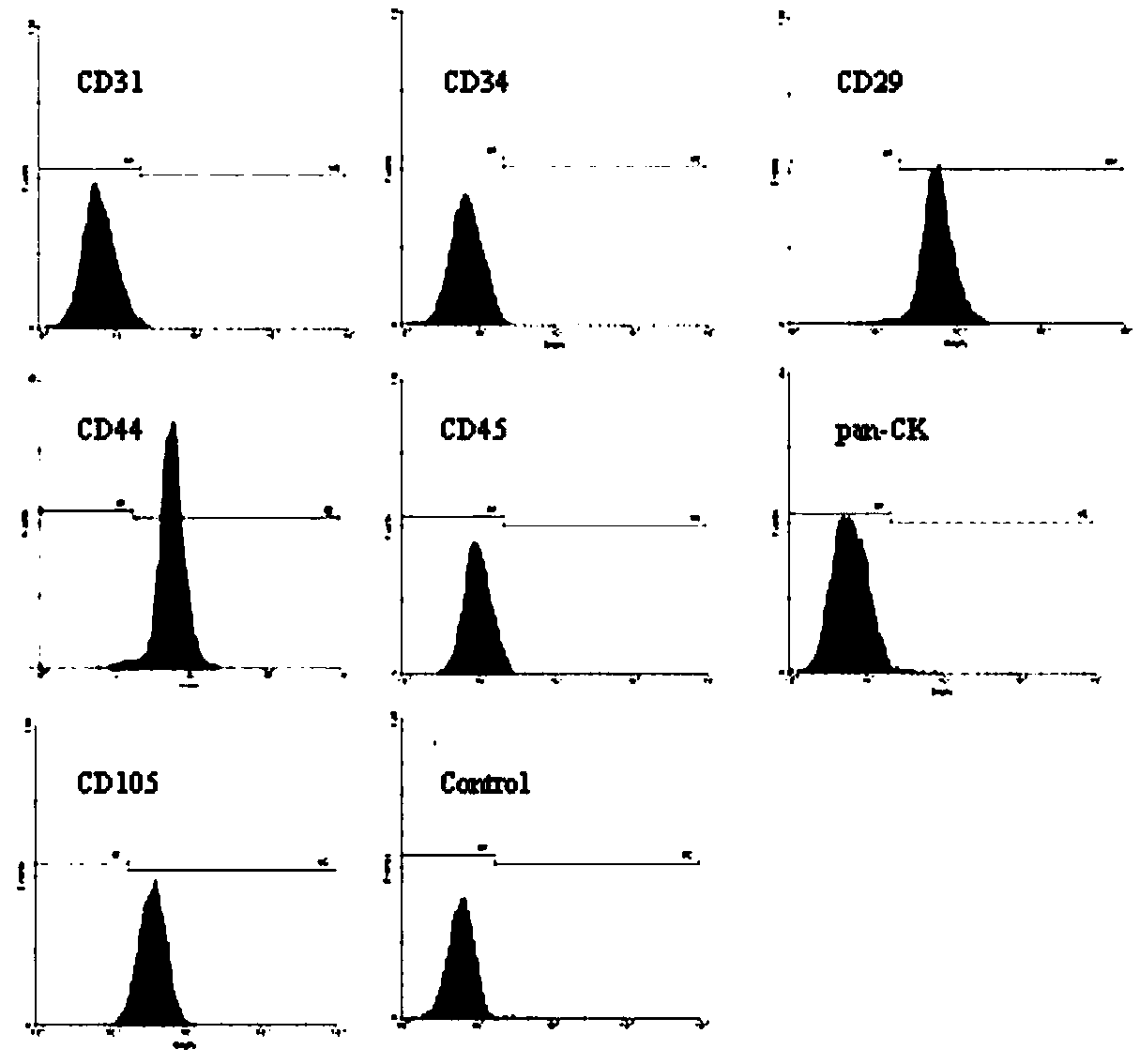
[0084] Embodiment 3 is identified to the AMSC that embodiment 1 obtains
[0085] The identification of AMSC mainly depends on the comprehensive identification of its morphology, cell surface molecular markers and differentiation ability. Obtaining high-quality AMSCs is a prerequisite for obtaining good clinical efficacy and ensuring patient safety.
[0086] 3.1 Immunophenotype of AMSC detected by flow cytometry
[0087] (1) Take the P3 passage cells and digest them with 0.125% trypsin, wash once with PBS containing 0.5% BSA, discard the supernatant, resuspend in PBS containing 0.5% BSA, and add the following primary antibodies: mouse anti-human The monoclonal antibodies CD11a, CD11b, CD29, CD31, CD34, CD44, CD45, CD105, HLA-DR, Pan-CK were incubated at 4°C for 30 min.
[0088] (2) Wash twice with PBS containing 0.5% BSA, add FITC-labeled goat anti-mouse secondary antibody, and incubate at 4°C for 30 min.
[0089] (3) Wash twice with PBS containing 0.5% BSA, suspend the cell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com