Application of aspirin in preventing or treating colon cancer
A technology of aspirin and colon cancer, applied in the field of biomedicine, to achieve the effect of reducing the degree of slowing down
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1, the experiment of aspirin to colon cancer xenograft tumor microenvironment influence
[0043] 1. Experimental materials
[0044] Experimental animals: BALB / C nude mice, 6-8 weeks old, female, weighing 25-30 g.
[0045] Experimental cells: human colorectal cancer HCT116 cell line, placed in 10% fetal bovine serum RPMI1640 culture medium, 37 ° C, 5% CO 2 Cultured, subcultured once every 2-3 days, and the cells in the logarithmic growth phase were taken for experiments.
[0046] Drug preparation: (1) Aspirin, dissolved in 0.9% normal saline, prepared as a 1.0 mg / ml solution. (2) Ticagrelor, dissolved in 500ml of 0.9% normal saline, prepared as a 1mg / ml solution.
[0047] 2. Experimental group
[0048] 1. Blank group: nude mice not inoculated with tumor + no drug treatment
[0049] 2. Control group: transplanted tumor + no drug treatment
[0050] 3. Aspirin administration group: transplanted tumor + intragastric administration of aspirin before, during a...
Embodiment 2
[0055] Embodiment 2, mouse body weight changes
[0056] 1. Experimental method
[0057] Feeding and drug treatment were carried out according to the experimental settings, and the body weight changes of the mice were measured and recorded. 2. Experimental results
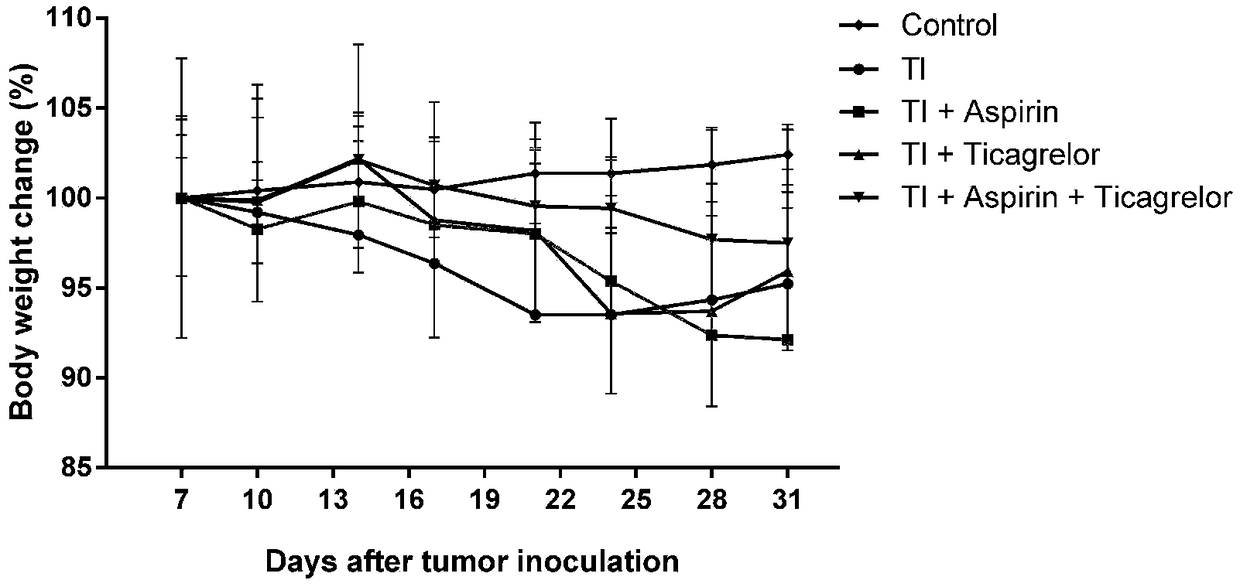
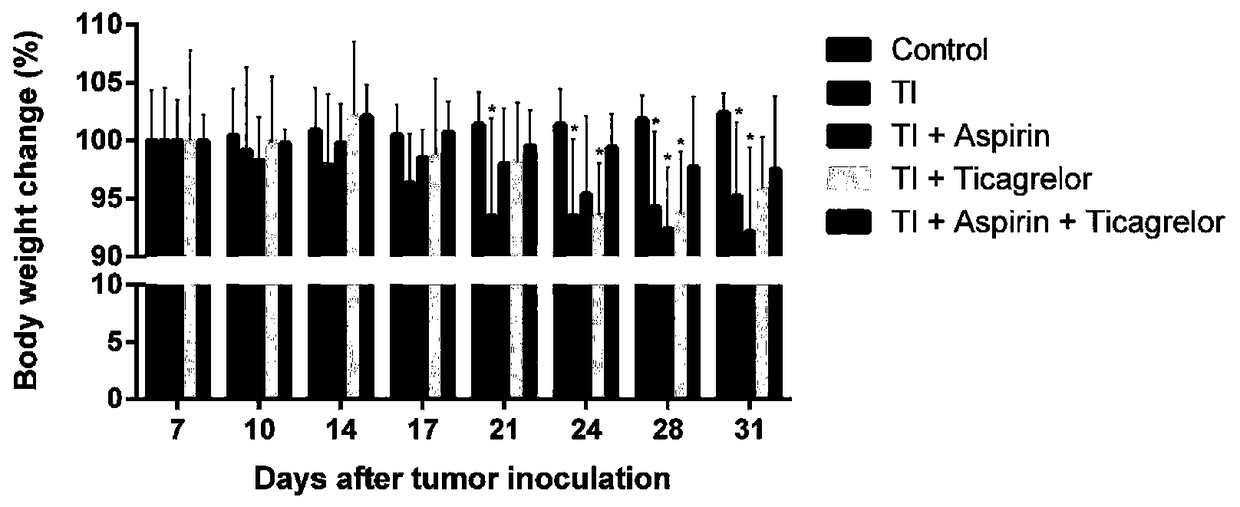
[0058] figure 1 , figure 2 is the body weight change of mice in each group 7-31 days after tumor inoculation.
[0059] The results showed that, compared with the normal mice in the blank group, there was no significant difference in the body weight of the mice in each group at 7-17 days after tumor inoculation (p>0.05); at 21 days, the body weight of the control group (TI) was significantly reduced (p0.05).
Embodiment 3
[0060] Example 3, Morphological and functional evaluation of transplanted tumor microvessels
[0061] 1. Experiment preparation
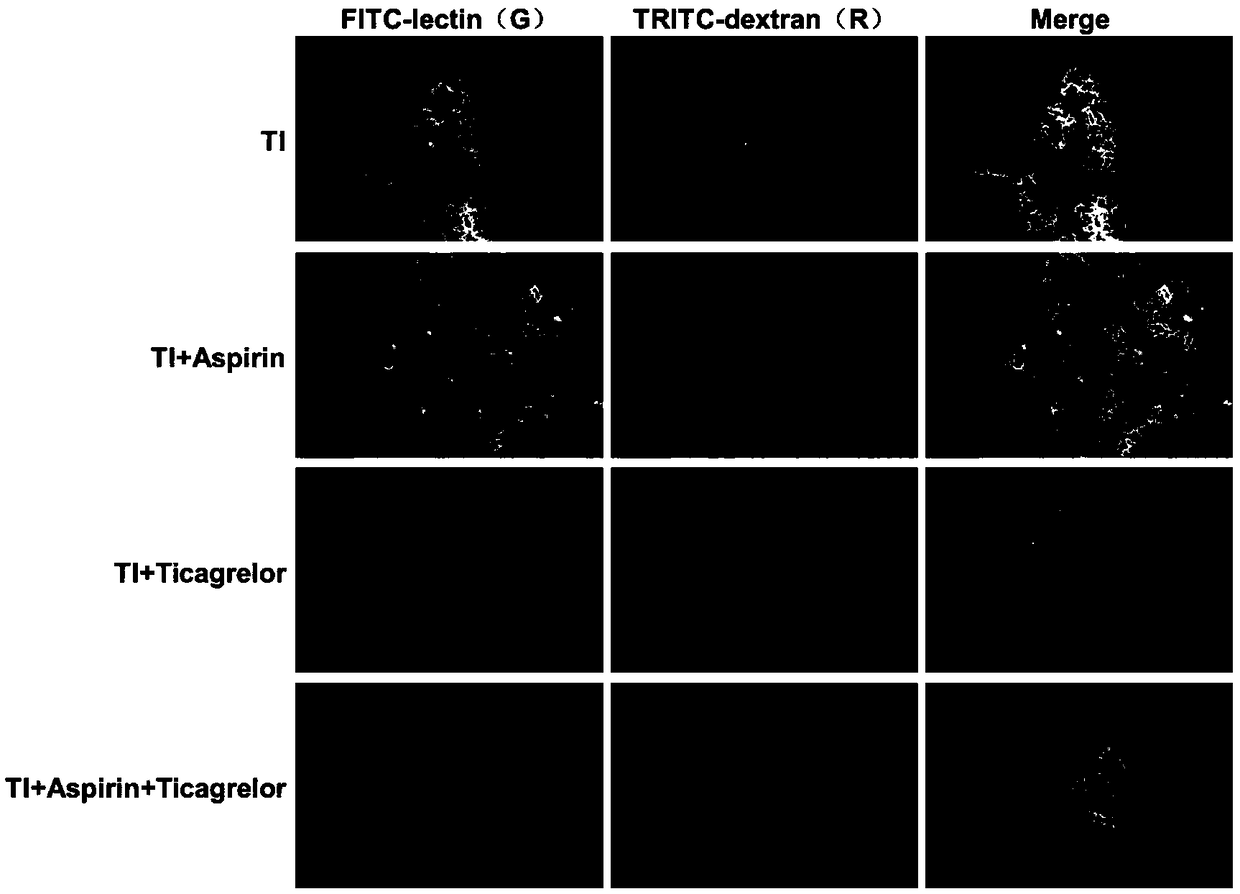
[0062] Six nude mice were randomly selected in each experimental group. TRITC-dextran 100mg / kg was injected into the tail vein. 3 hours later, FITC-lectin 10mg / kg was injected into the tail vein.
[0063] 2. Experimental method
[0064] 10 minutes after the drug injection, the mice were sacrificed by dislocation of the cervical spine, the tumor mass was taken out, and frozen sections (6 μm) were immediately observed under a laser confocal microscope, and 5 non-repetitive sections were randomly selected from each section under a high-power lens (×400). The image was collected from the field of view, the vascular morphology was evaluated by FITC-lectin green fluorescence, and the vascular permeability was evaluated by the ratio of the area of TRITC-dextran leaking out of the blood vessel to the total area.
[0065] 3. Experimental results
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com