Method of recycling butadiene by alkyne selective hydrogenation
A technology for selective hydrogenation and butadiene, applied in chemical recovery, purification/separation of hydrocarbons, chemical instruments and methods, etc., can solve problems such as easy aggregation, poisoning and deactivation of active components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
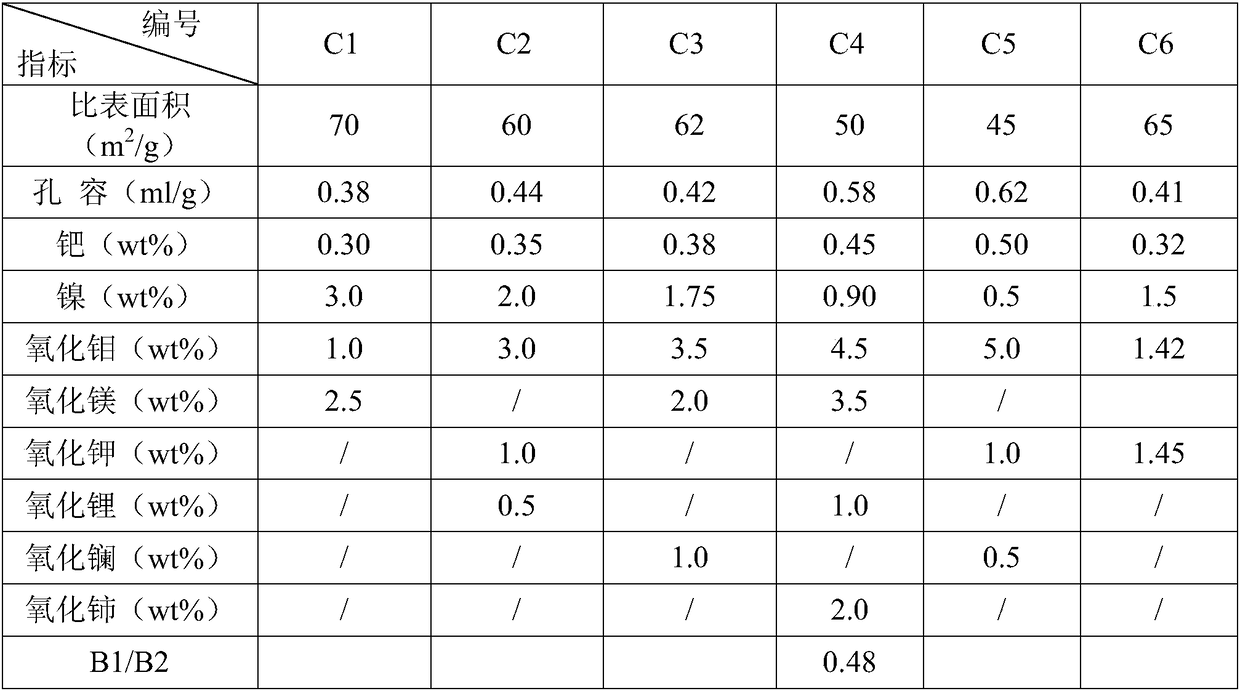
[0054] Embodiment uses the preparation of catalyst 1~6
[0055] Preparation of Catalyst C1:
[0056] Concentrate 4L to 50g Al 2 o 3 / L of sodium metaaluminate solution is placed in a stainless steel container with a stirrer and gas can be introduced into the bottom of the tank, the nickel nitrate solution is prepared and placed in a high-position container, a mixed gas of carbon dioxide and air is introduced, and the peristaltic pump is started at the same time Control the flow rate and drop the prepared nickel nitrate solution, the carbon dioxide concentration in the mixed gas is 70v%, and the flow rate is 3Nm 3 / h, the reaction temperature is 35°C, the pH value at the end of the reaction is 9.5, stop feeding carbon dioxide, age for 30 minutes, filter and separate the mother liquor, wash, and dry at 120°C for 5 hours to obtain nickel-containing pseudo-boehmite.
[0057] Mix and knead nickel-containing pseudo-boehmite with nitric acid and water, extrude into strips, dry at ...
Embodiment 1
[0083] The C4 fraction rich in alkynes is diluted with the C4 raffinate, and the weight ratio of the C4 fraction rich in alkynes to the C4 raffinate is 1:1. The adiabatic reactor adopts a single-stage adiabatic bubbling bed, using catalyst C1, and the catalyst is reduced at 120°C for 6h under a hydrogen atmosphere. The reaction inlet temperature is 45°C, the reaction pressure is 1.0MPa, and the liquid space velocity is 10h -1 , the molar ratio of hydrogen to alkyne is 3.0, Table 2 is the composition of materials before and after the reaction.
[0084] Material composition before and after table 2 reaction
[0085]
Embodiment 2
[0087] Dilute the alkyne-rich C4 fraction with raffinate C4 and cracked C4, and the weight ratio of the alkyne-rich C4 fraction to (raffinate + cracked C4) is 1:2. The adiabatic reactor adopts a single-stage adiabatic bubbling bed, using catalyst C2, and the catalyst is reduced at 120°C for 6 hours under a hydrogen atmosphere. The reaction inlet temperature is 30°C, the reaction pressure is 1.5MPa, and the liquid space velocity is 15h -1 , the molar ratio of hydrogen to alkyne is 3.0, Table 3 is the composition of materials before and after the reaction.
[0088] Table 3 Composition of materials before and after reaction
[0089]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com