Selective hydrogenation method
A selective, hydrogenation catalyst technology, applied in the direction of selective hydrorefining, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., can solve problems such as catalyst activity decline
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
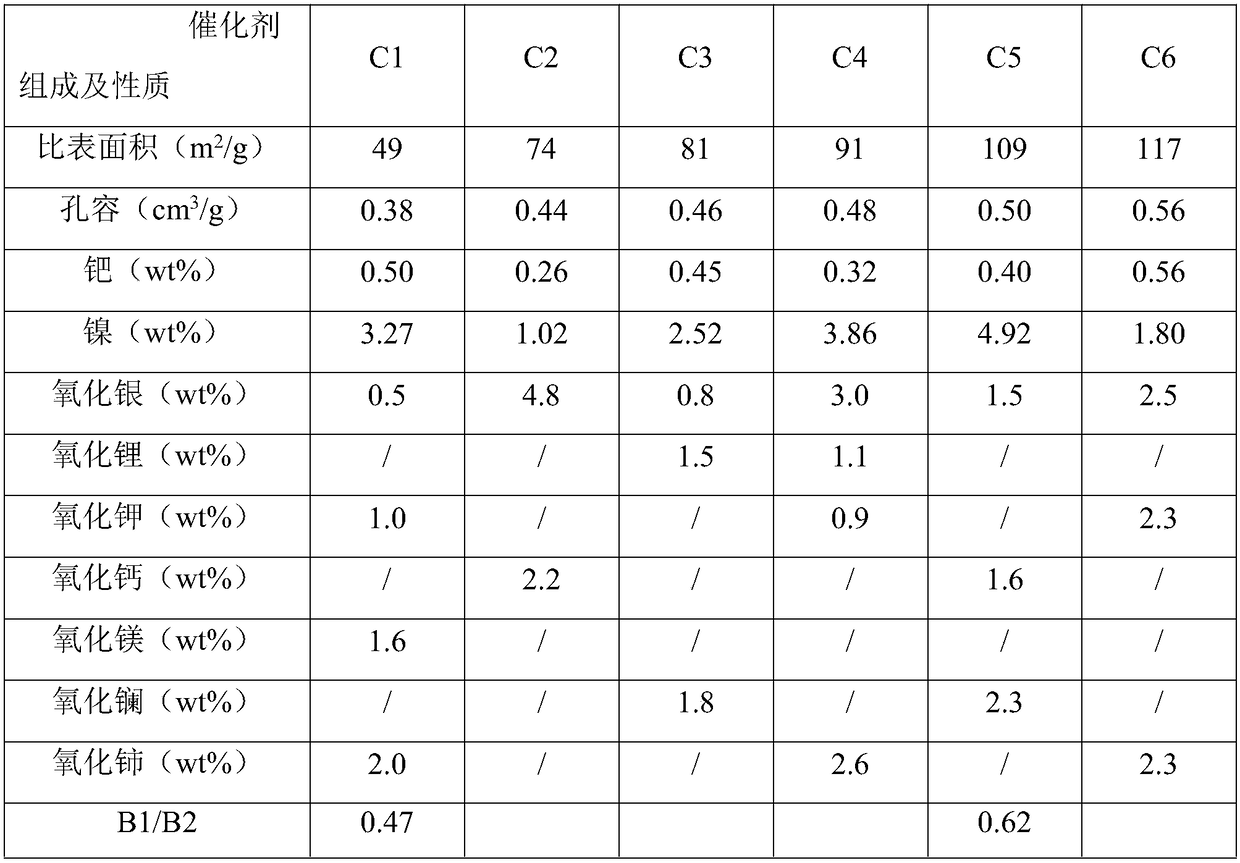
[0072] The preparation of embodiment with catalyst C1-C6:
[0073] Preparation of Catalyst C1:
[0074] Concentrate 5L to 60g Al 2 o 3 / L of sodium metaaluminate solution is put into a stainless steel container with a stirrer and the bottom of the tank can be fed with gas, and then a certain concentration of nickel nitrate solution is put into a high-position container, and the flow rate of the nickel nitrate is controlled by a peristaltic pump. The solution is added to the sodium metaaluminate solution, and a mixed gas of carbon dioxide and air is introduced into the stainless steel container at the same time, the concentration of carbon dioxide in the mixed gas is 70v%, and the flow rate is 3Nm 3 / h, the gelling temperature is 48°C, the pH value of the gelling is 9.3, after the gelling is completed, stop feeding carbon dioxide, age for 60 minutes, and the aging temperature is 50°C, filter and separate the mother liquor, wash, and dry at 120°C for 8 hours to obtain nickel-c...
Embodiment 1
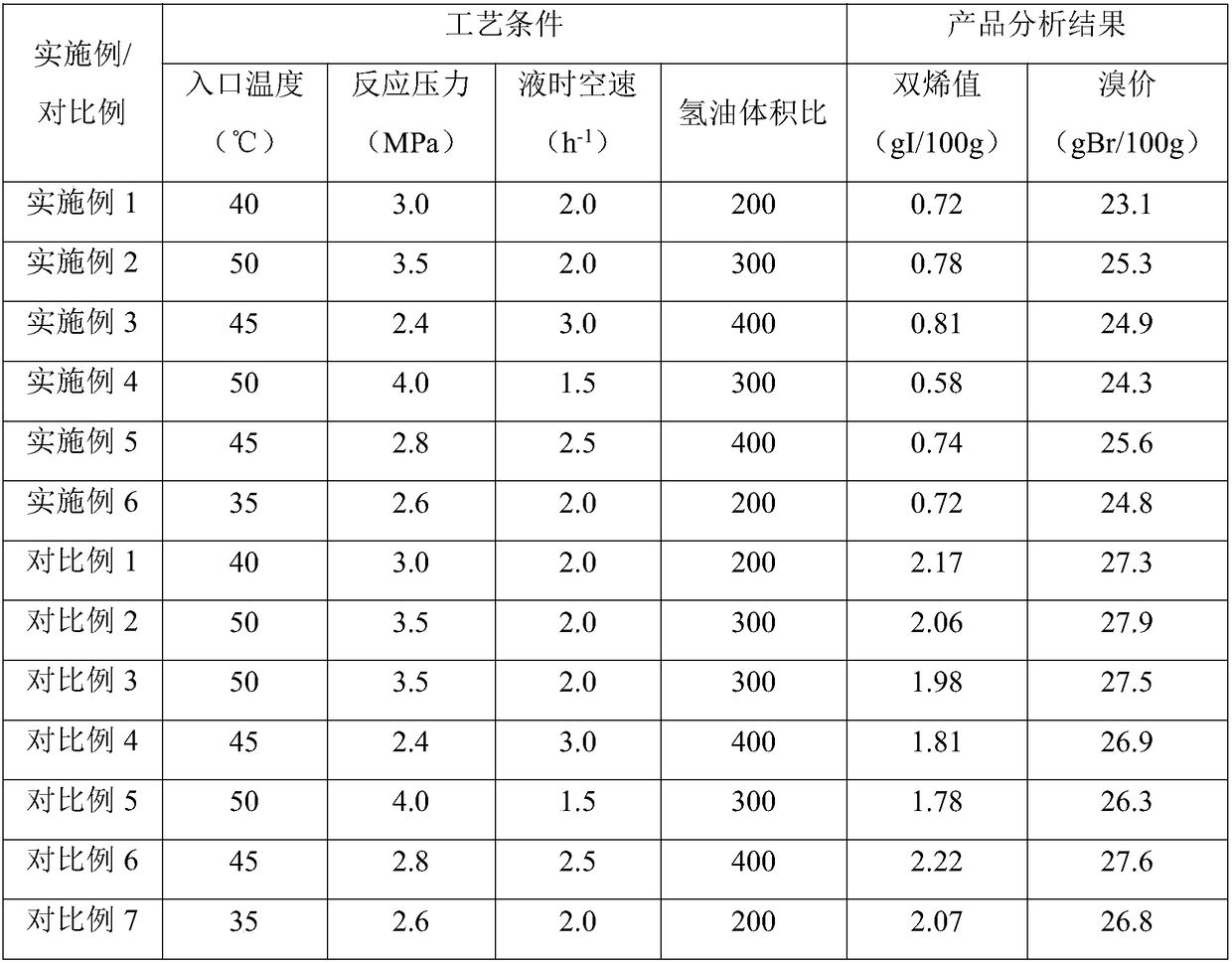
[0103] Put the catalyst 1 into a 250mL adiabatic fixed-bed reaction device, first carry out the reduction and activation of the catalyst, and maintain it for 8 hours under the conditions of a pressure of 2.8MPa, a bed temperature of 110°C, and a hydrogen flow rate of 125L / h to complete the reduction and activation of the catalyst.
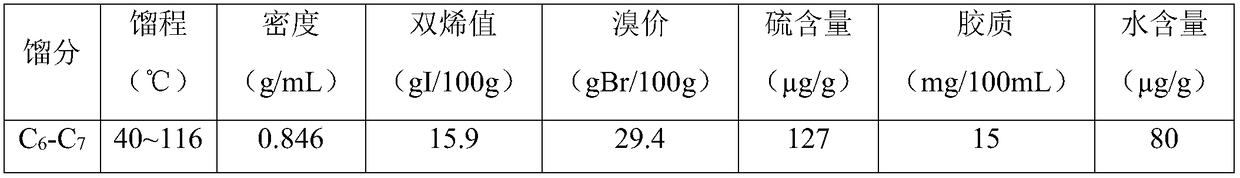
[0104] After the restore is complete, use C 6 -C 7 The distillate is used as raw material, at an inlet temperature of 40°C, a reaction pressure of 3.0MPa, and a liquid hourly space velocity of 2.0h -1 1. Operate for 200 hours under the condition of hydrogen oil volume ratio of 200:1, and sample and analyze the diene value and bromine value in the product every 24 hours. The average value of the analysis results is shown in Table 3.
Embodiment 2
[0108] Catalyst 2 was loaded into a 250mL adiabatic fixed-bed reactor, and the catalyst reduction and activation treatment method was the same as in Example 1.
[0109] After the restore is complete, use C 6 -C 7 The distillate is used as raw material, at an inlet temperature of 50°C, a reaction pressure of 3.5MPa, and a liquid hourly space velocity of 2.0h -1 1. Run for 200 hours under the condition of hydrogen oil volume ratio 300:1, sample and analyze the diene value and bromine value in the product every 24 hours, and the average value of the analysis results is shown in Table 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com