Method for simultaneously quantitative detecting erucyl amide and ethylene bis stearamide
A technology of ethylene bisstearamide and erucamide, which is applied in the field of instrument analysis, can solve the problems of cumbersome operation, low response of ultraviolet absorption signals, and low sensitivity, and achieve the effects of simple and fast operation, broad application prospects, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
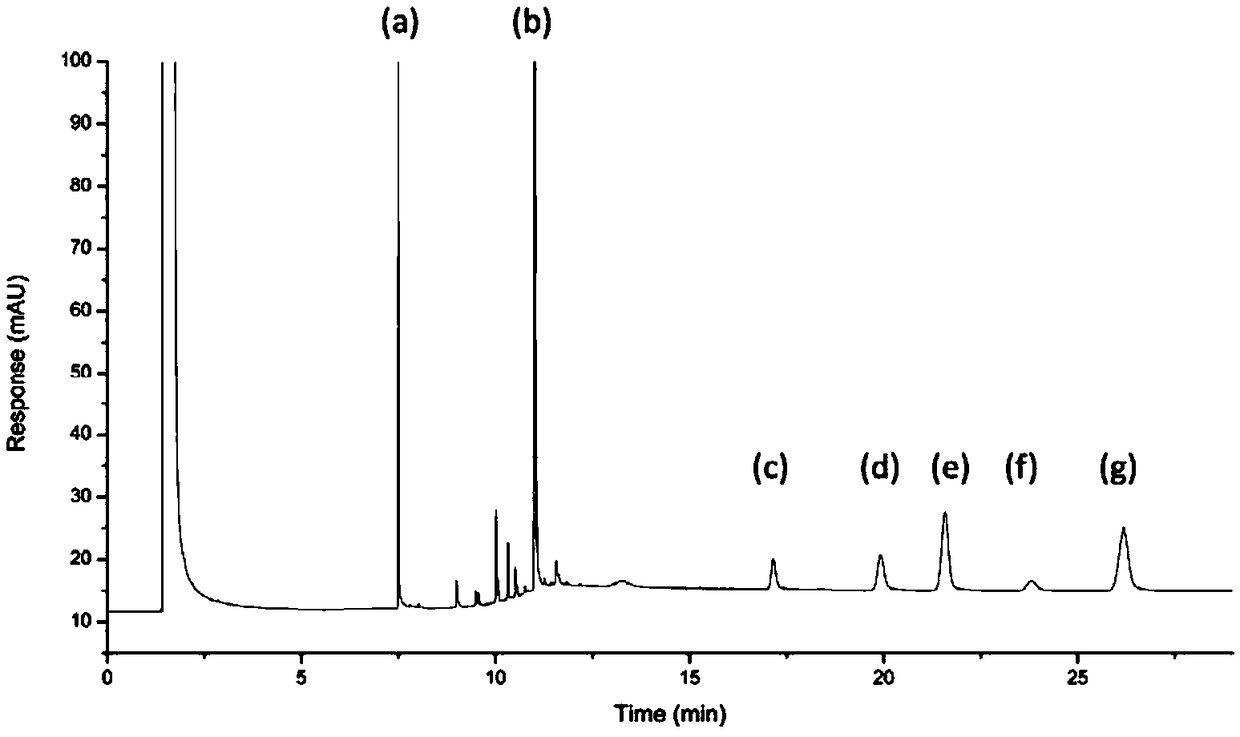
[0028] Gas chromatography analysis conditions include:
[0029] Adopt Agilent 7890B gas chromatograph, configure hydrogen flame ionization detector; adopt capillary gas chromatography column HP-5 (30m×320μm×0.25μm); inlet temperature: 300°C; detector temperature: 320°C; temperature program ( Column temperature): the initial temperature is 100°C, keep for 5min, then increase to 320°C at a rate of 50°C / min, and keep for 20min; the carrier gas flow rate is 2.0ml / min; the split ratio is 2:1; the injection volume: 2μl.
[0030] In addition, a mixed solvent: dichloromethane-methanol (volume ratio 9:1) was used.
[0031] Prepare internal standard stock solution (1mg / ml): take 50mg of dimethyl phthalate, accurately weigh it, place it in a 50ml volumetric flask, dilute to the mark with a mixed solvent, and obtain it;
[0032] Prepare the control stock solution (0.1mg / ml): Take 10mg of ethylene bisstearamide and erucamide respectively, accurately weigh them, put them in a 100ml volumet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com