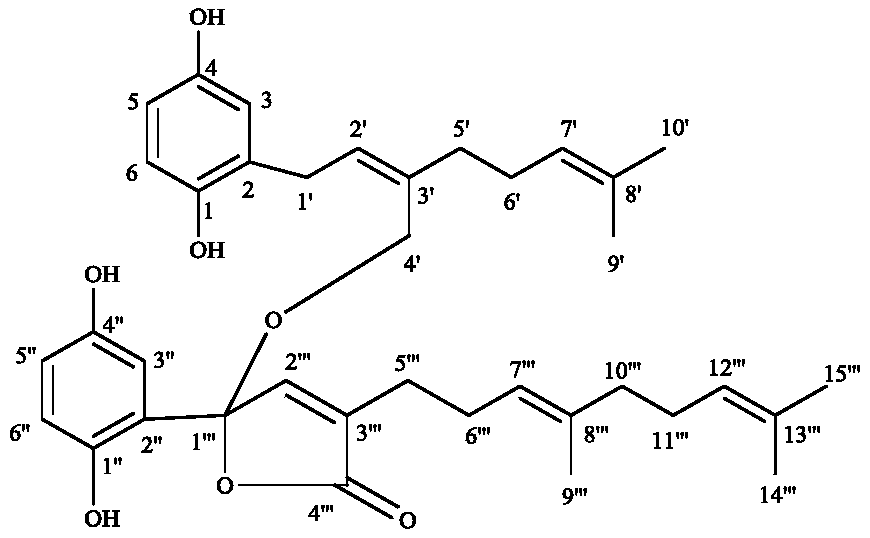
Meroganocochlearin, pharmaceutical composition thereof and application of pharmaceutical composition
A mixed-source terpene dimer and compound technology, applied in the field of pharmacy, can solve the problems of unreported pharmacological effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] G. cochlear fruiting bodies (20kg) were crushed and extracted four times with 150L of 95% ethanol, once every 3 days, the extract was concentrated to 4L by vacuum distillation, and then extracted four times with ethyl acetate. The ester layer was concentrated under reduced pressure to obtain 850 g of extract. The extract was eluted with a silica gel column (chloroform: methanol, V / V, 100:0 to 0:100) to obtain 11 fragments I-XI. The VII was eluted by medium pressure column chromatography (MeOH:H 2 O, V / V, 20:80, 40:60, 60:40, 80:20, 100:0) to obtain fragments A-E. Put fragment D by LH-20 (methanol), LH-20 (acetone), normal phase silica gel column chromatography to obtain the crude meroganocochlearin, and then pre-HPLC (MeOH-H 2 O, MeCN-H 2 O) The 98% pure compound meroganocochlearin is obtained. The identification of the compound structure is determined by the comparison and analysis of spectral data. See Table 1 for specific nuclear magnetic data
[0026] Table 1. NMR...
Embodiment 2
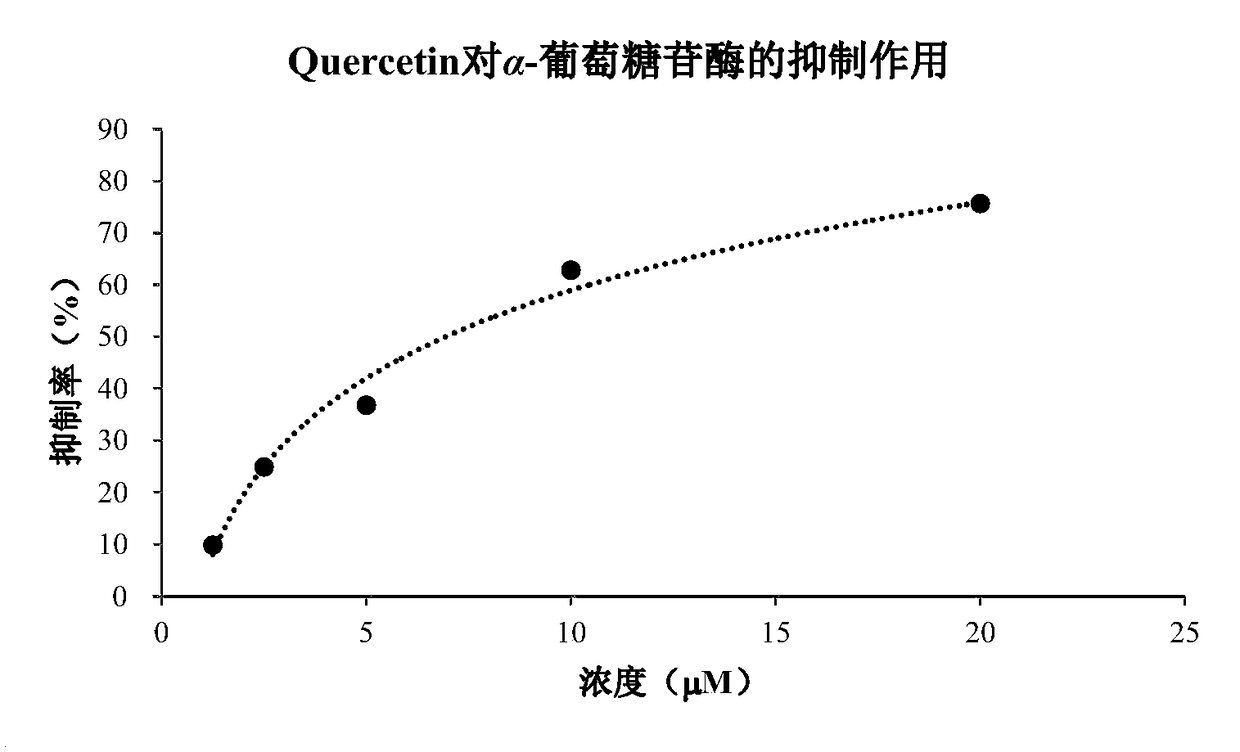
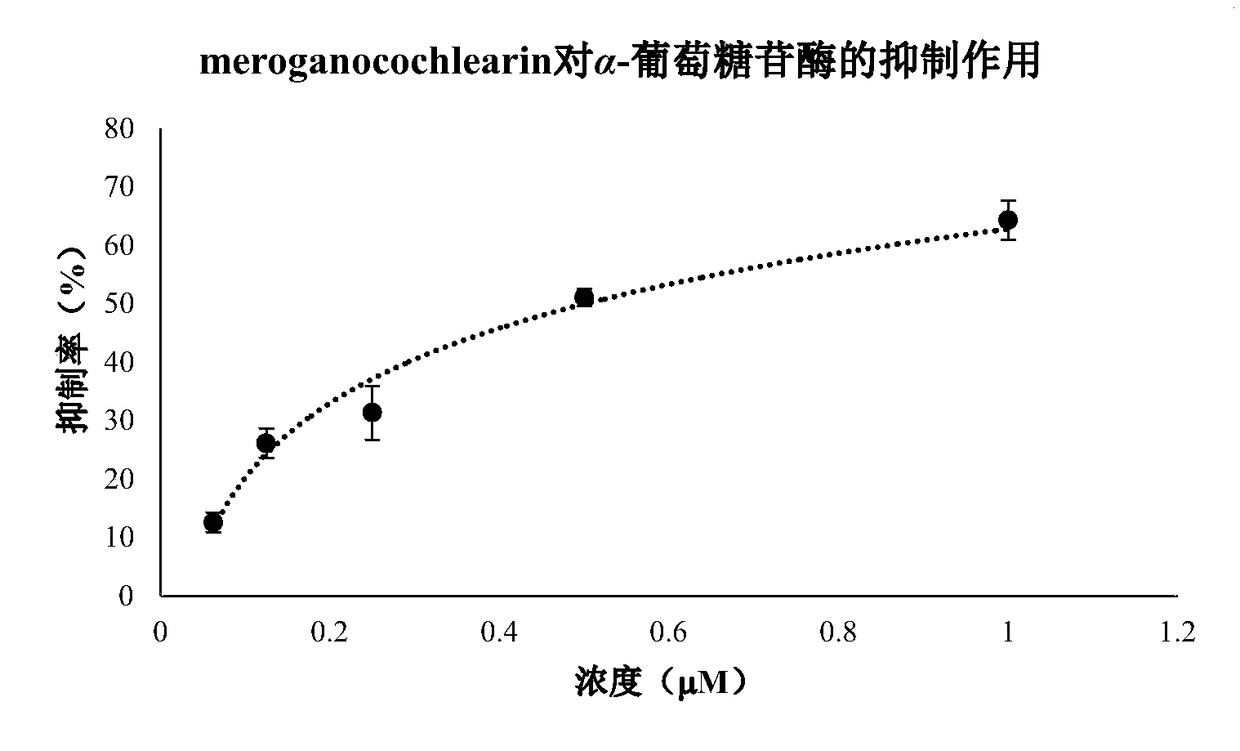
[0029] Inhibition experiment of compound meroganocochlearin on α-glucosidase activity:
[0030] Experimental reagents: α-glucosidase, 4-Nitrophenylα-D-glucopyranoside, and positive control Quercetin were purchased from Sigma.
[0031] Experimental method: add different concentrations of the test compound and α-glucosidase solution (final concentration 0.025U / ml), buffer, and substrate (final concentration 1mM) into 96-well microtiter plate in sequence, and mix well. The concentration is set to 3 repeat wells. At the same time, a blank control without drugs and a quercetin positive control were set. Incubate at 37°C for 50 minutes, measure the OD value at 405 nm with a microplate reader, and calculate the inhibition rate of α-glucosidase activity.
[0032] α-Glucosidase inhibition rate (%) = (1-test hole OD 405nm / Control well OD 405nm )×100%
[0033] Experimental results: The compound meroganocochlearin has a strong inhibitory effect on α-glucosidase, and it is concentration-depen...
Embodiment 3
[0036] Inhibition experiment of compound meroganocochlearin on pancreatic lipase activity:
[0037] Experimental reagents: Pancreatic lipase (PPL), p-nitrophenyl butyrate (p-NPB), Tris, positive control Orlistat were purchased from Sigma; D-PBS was purchased from Hyclone; Anhydrous calcium chloride was a chemical reagent from Chengdu Kelon Factory products.
[0038] Experimental method: On a 96-well microtiter plate, mix the test compounds of different concentrations with the PPL solution (final concentration 1.25U / mL) thoroughly, set 3 replicate wells for each concentration, 37℃, 15min; add p -NPB (final concentration 0.5mM), mix well, 37℃, 15min; BioTek PowerWave XS microplate reader measures OD400nm / 630nm value, the detection wavelength is 400nm, and the reference wavelength is 630nm. The experiment set up blank control wells and Orlistat positive control wells at the same time.
[0039] Inhibition rate of PPL activity (%) = (1-experimental well OD 400nm / 630nm / Control well OD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com