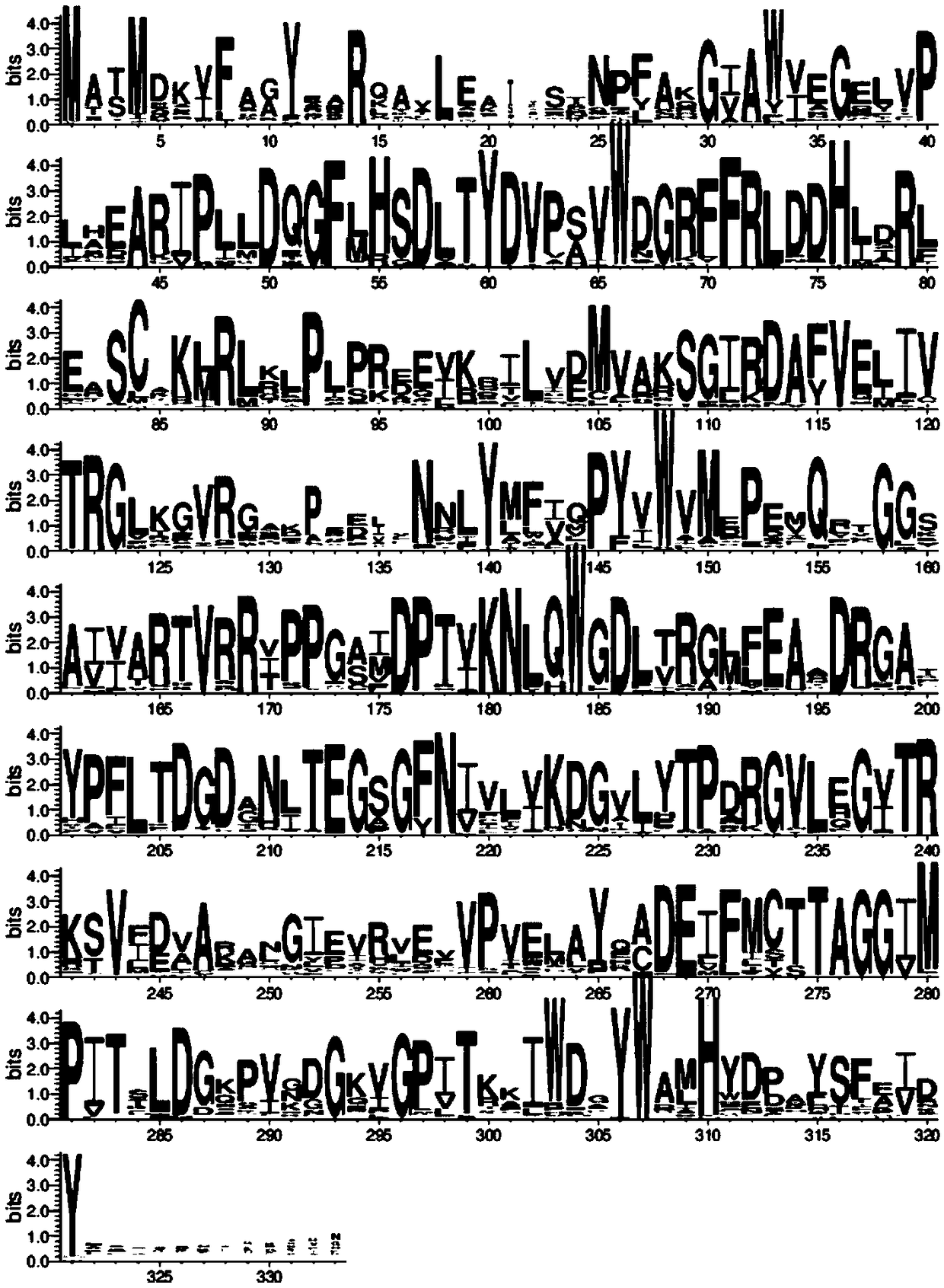Omega-transaminase mutant and application thereof
A mutant and transaminase technology, applied in the field of molecular biology, can solve the problems of half-inactivation temperature and half-life to be further improved, and achieve the effects of improving experimental efficiency and feasibility, improving thermal stability, and increasing the probability of positive mutation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] 1. Sequence Consistency Analysis
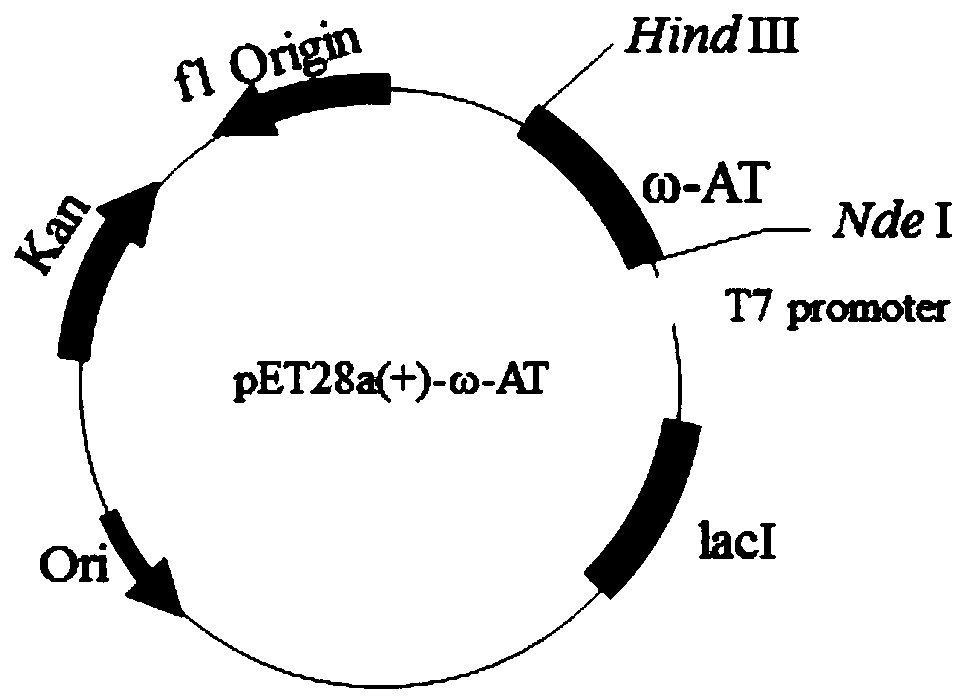
[0050] According to the ω-transaminase gene sequence of Aspergillus terreus in the NCBI database (Genbank: XM_001209325) and the codon usage frequency distribution table of Escherichiacoli in the codon usage data (http: / / www.kazusa.or.jp / codon / ), analyze ω - Codon usage for transaminases. Codon-optimized ω-transaminase gene (ω-opt-TA gene), its nucleotide sequence is shown in SEQ ID NO.3, its encoded protein has a total of 325 amino acids, and its amino acid sequence is shown in SEQ ID NO.4 .
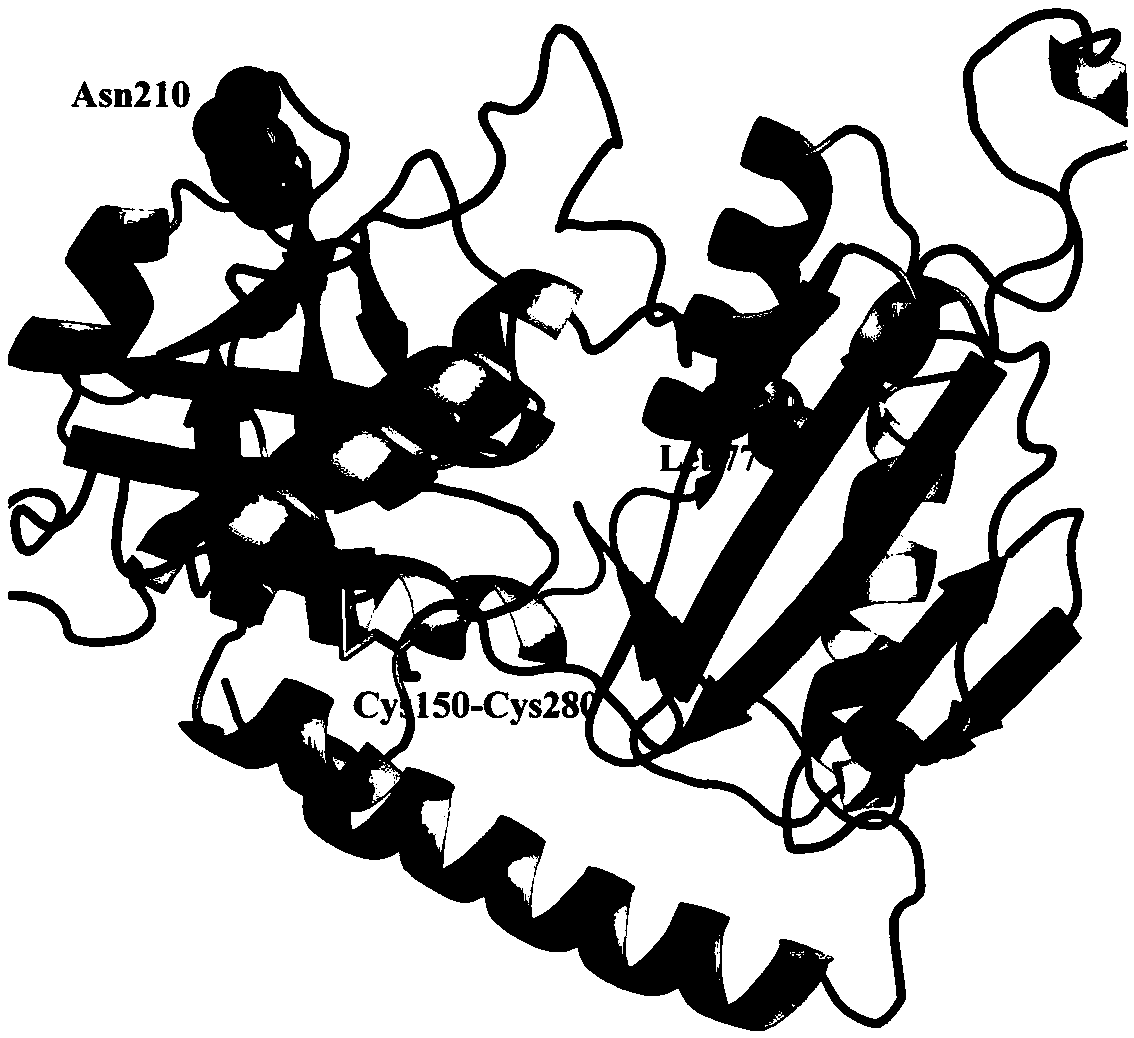
[0051] Through BLAST comparison, set the maximum value of E-value to 10 -3 , the sequence redundancy does not exceed 0.9, and a total of 91 homologous amino acid sequences were screened. Mutation sites were screened based on sequence consistency. The principle of screening was that the conservation threshold of mutations was 0.6, that is, the amino acid type of this site accounted for more than 60% in the multiple sequence alignment, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com