Alkaline pullulanase transformed in site-specific mutagenesis mode, and application thereof
A technology of pullulanase and site-directed mutation, which is applied in the fields of application, enzyme, hydrolase, etc., can solve the problems of limited application, low pullulanase secretion ability, and difficulty in meeting the conditions of industrial production, and achieve improved thermal stability , Enzyme activity and stability improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1 Construction of pullulanase mutant F744A
[0018] According to the 3D structure analysis of alkaline pullulanase PulSL3△C ( figure 1 ), the 744th phenylalanine leads to a wide crack and a sharp bulge, this structure may inhibit the enzyme from hydrolyzing the second α-1, 6 glycosidic bond of pullulan, and to the branched chain The hydrolysis inhibition of densely branched sugars such as starch and glycogen was more serious. The side chain of phenylalanine is a benzene ring with a large steric hindrance, which leads to the generation of cracks and protrusions. If Phe744 is mutated into alanine with a methyl side chain, namely Ala744, the benzene ring can be greatly reduced The resulting steric hindrance. This mutant was named F744A.
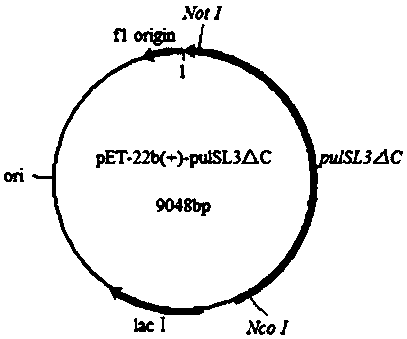
[0019] Design primers containing mutation sites to recombine plasmid pET-22b(+)- pulSL3△C ( figure 2 ) as a template, the expression plasmid pET-22b(+)- F744A , transforming E. coli BL21(DE3) competent cells were used...
Embodiment 2
[0027] Example 2 Induced expression and purification of pullulanase mutant F744A
[0028] A single colony was picked from the transformation plate and inoculated into LB liquid medium containing 100 µg / mL Amp for overnight culture. Inoculate the overnight culture solution at 1% inoculum volume in 50 mL LB medium containing 100 µg / mL Amp, and cultivate to OD at 37°C at 180 rpm 600 When it reaches 0.5, add IPTG and 0.5% NaCl at a final concentration of 0.1 mmol / L, and incubate at 25°C for 32 h at 180 rpm. The fermentation broth was centrifuged at 13000 rpm for 10 min at 4°C, and the supernatant was taken as the crude enzyme solution.
[0029] The crude enzyme solution was first concentrated with a 10 kDa hollow fiber column, and then (NH 4 ) 2 SO 4 After fractionation precipitation, the dialyzed enzyme solution was filtered through a 0.22 µm membrane to prepare the loading sample. First use the prepacked column DEAE FF (Hi Trap TM , 5 mL) for purification, the equilibrium ...
Embodiment 3
[0030] Example 3 Determination of Enzyme Activity and Properties of Pullulanase Mutant F744A
[0031] 1. Enzyme activity assay of pullulanase mutant F744A
[0032]The activity of pullulanase was determined by DNS method. Incubate the mixture containing 0.9 mL 0.5% pullulan (TCI company) and 0.1 mL enzyme solution under appropriate conditions for 10 min, add 1.5 mL DNS to terminate the reaction, boil for 5 min to develop color, cool down and dilute to volume with distilled water 10 mL, measure OD 540 . Definition of enzyme activity unit: Under certain reaction conditions, the amount of enzyme required to hydrolyze pullulan to produce 1 μmol reducing sugar per minute is defined as an enzyme activity unit U.
[0033] Compared with the original enzyme PulSL3△C, the activity of pullulanase mutant F744A was increased by 32.18%.
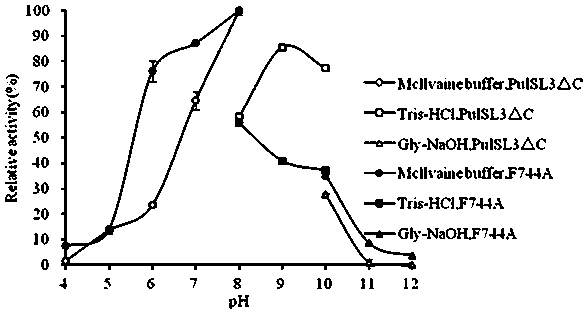
[0034] 2. Optimum reaction pH and pH stability of pullulanase mutant F744A
[0035] Determination of the optimum reaction pH: Put the pullulanase in t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com