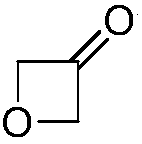
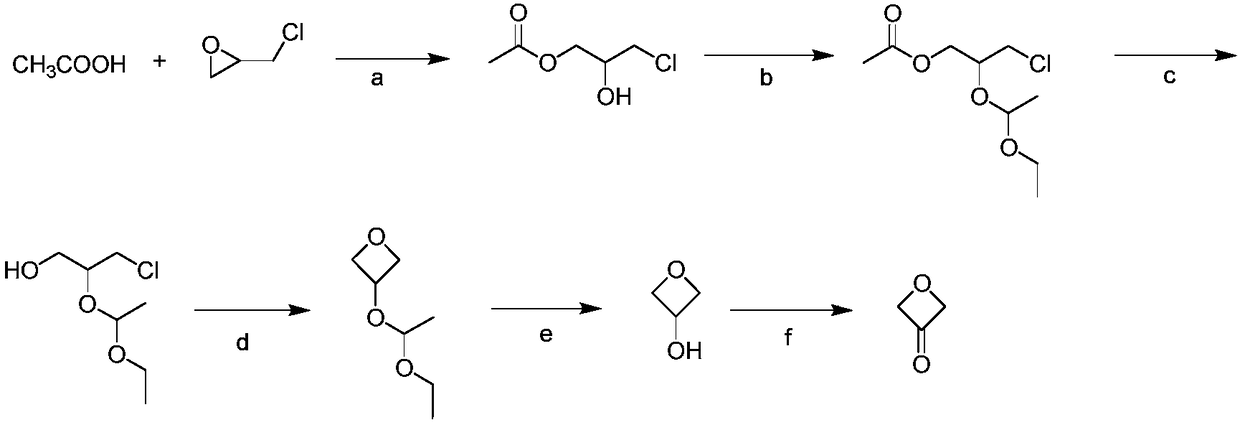
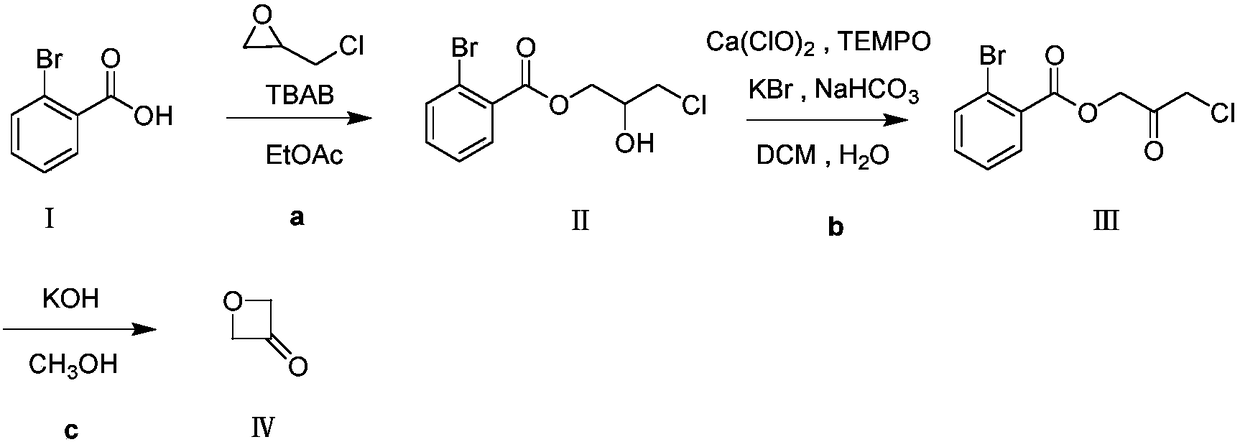
Preparation method for 3-oxetanone
A technology of oxetanone and oxidant, which is applied in the direction of organic chemistry, can solve the problems of low reaction selectivity, difficult separation and purification, difficult industrial production, etc., and achieve the effect of short synthetic route, high purity and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1.1 Preparation of compound Ⅱ
[0024] Dissolve 5.0g o-bromobenzoic acid in 20mL N,N-dimethylformamide, add 2.76g epichlorohydrin, then add 0.40g benzyltriethylammonium chloride, and react at 80°C. After reacting for 5 hours, TLC detected that the reaction of raw materials was complete. Evaporate N,N-dimethylformamide to dryness, extract with ethyl acetate, wash with appropriate amount of saturated NaHCO 3 The solution was washed twice, and then washed twice with water, and the organic phase was separated, anhydrous Na 2 SO 4 After drying, the solvent was distilled off to obtain 4.75 g of brownish-yellow liquid, compound II, with a yield of 65%. 1 H NMR: (500MHz, CDCl 3 ):δ=7.82(dd,J=7.4,2.1Hz,1H),7.68(dd,J=7.7,1.4Hz,1H),7.42–7.33(m,2H),4.52–4.45(m,2H), 4.28–4.20 (m, 1H), 3.80–3.67 (m, 2H), 2.80 (d, J=5.4Hz, 1H).
[0025] 1.2 Preparation of compound Ⅱ
[0026] Dissolve 5.0g o-bromobenzoic acid in 20mL N,N-dimethylformamide, add 3.45g epichlorohydrin, then add 0.4...
Embodiment 2
[0038] 2.1 Preparation of compound III
[0039] Dissolve 5.0g of 3-chloro-2-hydroxypropyl-2-bromobenzoate in 25mL of dichloromethane, add 25mL of water, add 2.23g of potassium bromide, 0.72g of sodium bicarbonate, 53mg of 2,2 , 6,6-tetramethylpiperidine-nitrogen-oxide (TEMPO), 14.6g of NaClO solution (13%) was slowly added dropwise at 20°C. After reacting for 2 hours, add Na equivalent to the oxidant to the system 2 SO 3 solution, stirred for 30min, extracted and separated, and the organic phase was washed successively with 1M HCl, saturated NaHCO 3 Solution, water wash once. Anhydrous Na for organic phase 2 SO 4 Dry and evaporate to dryness to obtain a crude product, which is beaten with petroleum ether: ethyl acetate = 3:1 to obtain 2.08 g of white solid, compound III, with a yield of 42%. 1 H NMR: (500MHz, CDCl 3 ):δ=7.87(dd,J=7.4,2.1Hz,1H),7.63(dd,J=7.6,1.5Hz,1H),7.37–7.27(m,2H),5.09(s,2H),4.18( s, 2H).
[0040] 2.2 Preparation of compound III
[0041] Dissolve 5...
Embodiment 3
[0051] Preparation of compound Ⅳ
[0052] Dissolve 40.0g of 3-chloro-2-oxopropyl-2-bromobenzoate in 200mL of methanol, add 12.07g of NaOH, and react at 80°C for 8h. The system was filtered, and the filtrate was distilled at atmospheric pressure first. After the solvent was distilled off, it was distilled under reduced pressure using a rectification column to collect fractions with stable boiling points to obtain 5.63 g of compound IV, a colorless oily liquid, with a yield of 57%. 1 H NMR: (500MHz, CDCl 3 ): δ=5.35(s, 4H).
[0053] 3.2 Preparation of Compound IV
[0054] Dissolve 40.0g of 3-chloro-2-oxopropyl-2-bromobenzoate in 200mL of methanol, add 16.90g of KOH, and react at 80°C for 8h. The system was filtered, and the filtrate was distilled at atmospheric pressure first. After the solvent was evaporated, it was distilled under reduced pressure using a rectification column to collect fractions with stable boiling points to obtain 6.72 g of a colorless oily liquid, namely...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com