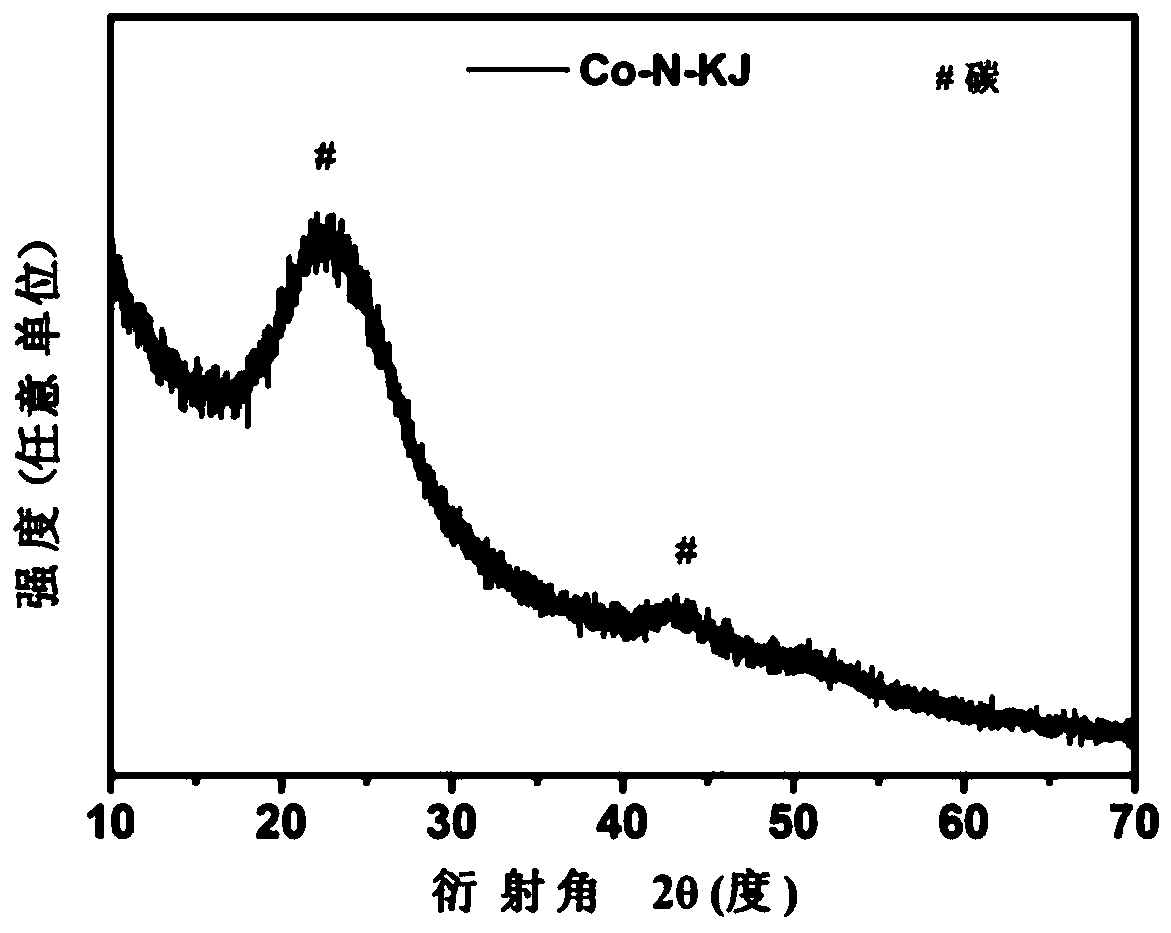
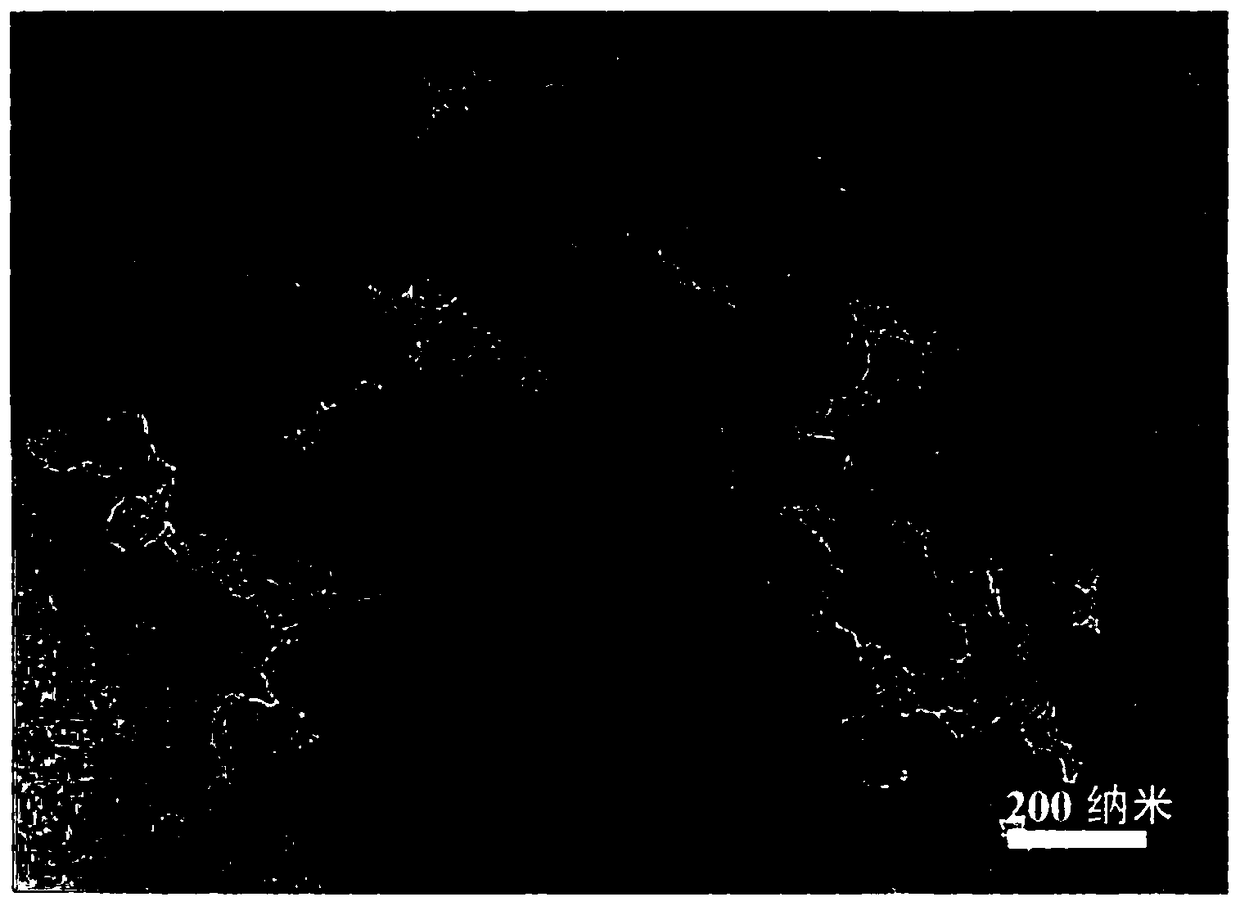
Non-noble metal catalyst used for catalytic oxygen reduction and preparation method thereof
A non-precious metal and catalyst technology, which is applied in the field of non-precious metal catalysts for catalyzing oxygen reduction and its preparation, can solve the problems of low atomic utilization rate of non-precious metal catalysts, inability to prepare large-scale and large quantities, and poor product consistency, and achieve excellent material transport. transport capacity, excellent oxygen reduction catalytic activity, and excellent electrical conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] To 1000g fruit shell activated carbon (purchased from Gongyi Meiyuan Water Purification Material Co., Ltd., the specific surface area is 1200m 2 / g) Pretreatment: In a 1000ml glass beaker, heat and wash the above-mentioned activated carbon with deionized water and 1M hydrochloric acid at about 80°C and soak for 15min, then put it in a vacuum drying oven at 80°C and dry it for later use.
[0072]In a 100ml glass beaker, 300mg of cobalt nitrate (purchased from Sinopharm Reagent Company) and 660mg o-phenanthroline (purchased from Sinopharm Reagent Company) were added to 50ml of ethanol for stirring and mixing to obtain the first mixture.
[0073] In a 100ml glass beaker, add 700mg of the above-mentioned processed fruit shell activated carbon into 50ml of ethanol for ultrasonic (microwave ultrasonic instrument, purchased from Hangzhou Farland Ultrasonic Company) and stirring (magnetic stirrer, purchased from Hefei Kejing Instrument Company) Processing yielded a uniformly di...
Embodiment 2
[0085] According to the method described in Example 1, 1500g fruit shell activated carbon (purchased from Gongyi Meiyuan Water Purification Material Co., Ltd., the specific surface area is 1500m 2 / g) for pretreatment, for subsequent use.
[0086] In a 500ml glass beaker, 600mg of ferric nitrate (purchased from Sinopharm Reagent Company) and 1200mg of 2,2-bipyridyl (purchased from Sinopharm Reagent Company) were added to 100ml of distilled water and stirred to obtain the first mixture.
[0087] In a 500ml glass beaker, 1000mg of the above-mentioned treated shell activated carbon was added to a mixture of 50ml of ethanol and 50ml of water for ultrasonic and stirring treatment to obtain a uniformly dispersed black second mixture.
[0088] In a 1000ml glass beaker, the second mixture was slowly added dropwise to the above first mixture at a rate of 70ml / min, and vigorously stirred (magnetic stirring, 300rpm) for 24h. The stirred and uniformly adsorbed black mixture was vacuum fi...
Embodiment 3
[0092] According to the method described in embodiment 1, to 1500g Super P active carbon (purchased from the special secret company of Switzerland, specific surface area is 1800m 2 / g) for pretreatment, for subsequent use.
[0093] In a 500ml glass beaker, 400mg of ferric nitrate and 800mg of phenanthroline were added into 100ml of acetone for stirring and mixing to obtain the first mixture.
[0094] In a 500ml glass beaker, 1000mg of the above-mentioned treated Super P activated carbon was added into 100ml of acetone for ultrasonic and stirring treatment to obtain a uniformly dispersed second mixture.
[0095] In a 1000ml glass beaker, the second mixture was slowly added dropwise to the above first mixture at a rate of 80ml / min, and vigorously stirred (magnetic stirring, 300rpm) for 24h. The stirred and uniformly adsorbed black mixture was vacuum filtered using a Buchner funnel, rinsed with deionized water, and then placed in a freeze-drying oven (-50°C) for drying. After 24...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com