A kind of on-line depolymerization method of by-product paraformaldehyde in the synthesis reaction of trioxane
A technology of paraformaldehyde and paraformaldehyde, which is applied in the preparation of organic compounds, carbon-based compounds, chemical instruments and methods, etc., can solve the problem of unblocking tubes, long maintenance and recovery time, and increased thermal resistance of heat exchanger fouling Large, large loss of raw materials and other problems, to achieve considerable economic and social benefits, reduce economic losses, the effect of mild reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
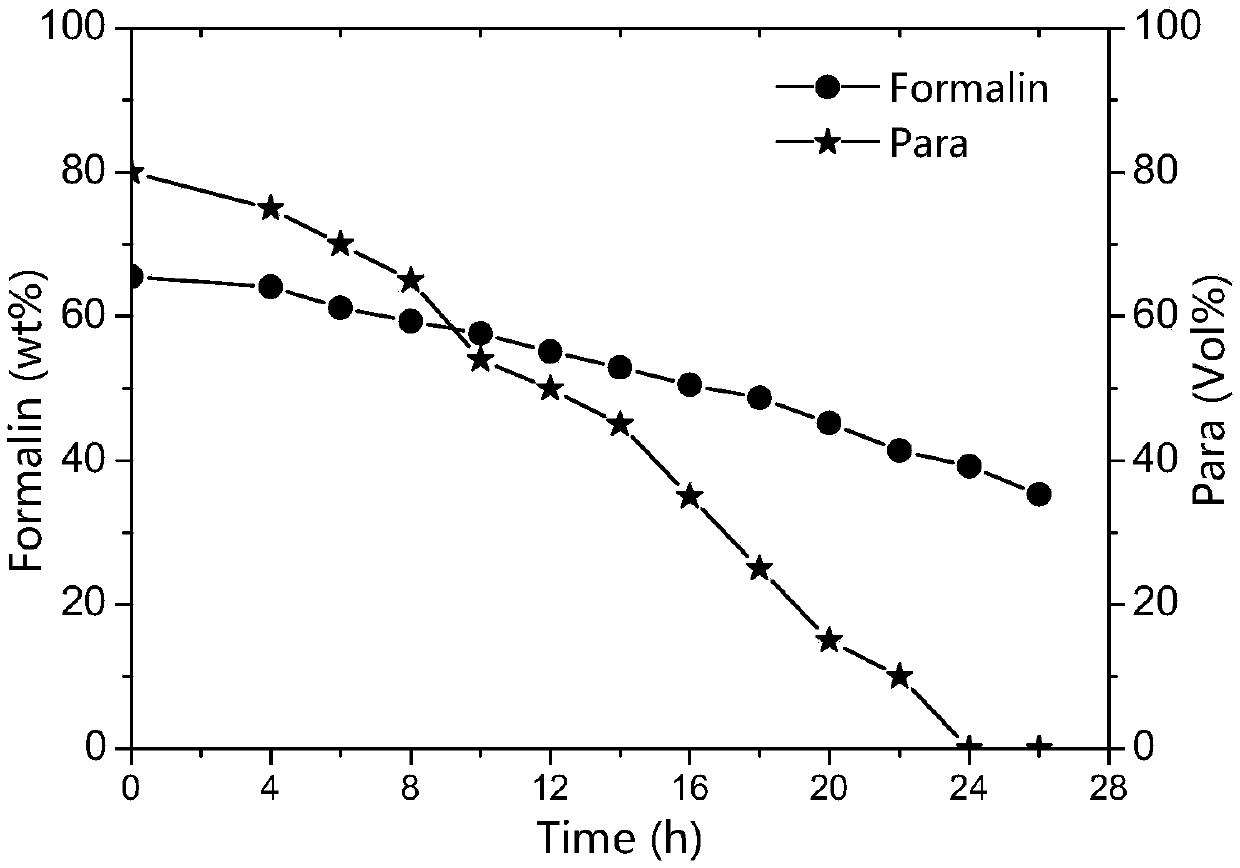
[0055] The paraformaldehyde synthesis reaction solution to be depolymerized: the main components are 65.5wt% formaldehyde and 8.5wt% paraformaldehyde, the reaction temperature is 120°C, the catalyst sulfuric acid concentration is 4.32wt%, the heating steam is 0.3MPa, take the reaction kettle liquid and let it stand The volume content of the by-product paraformaldehyde precipitation is 80Vol%.
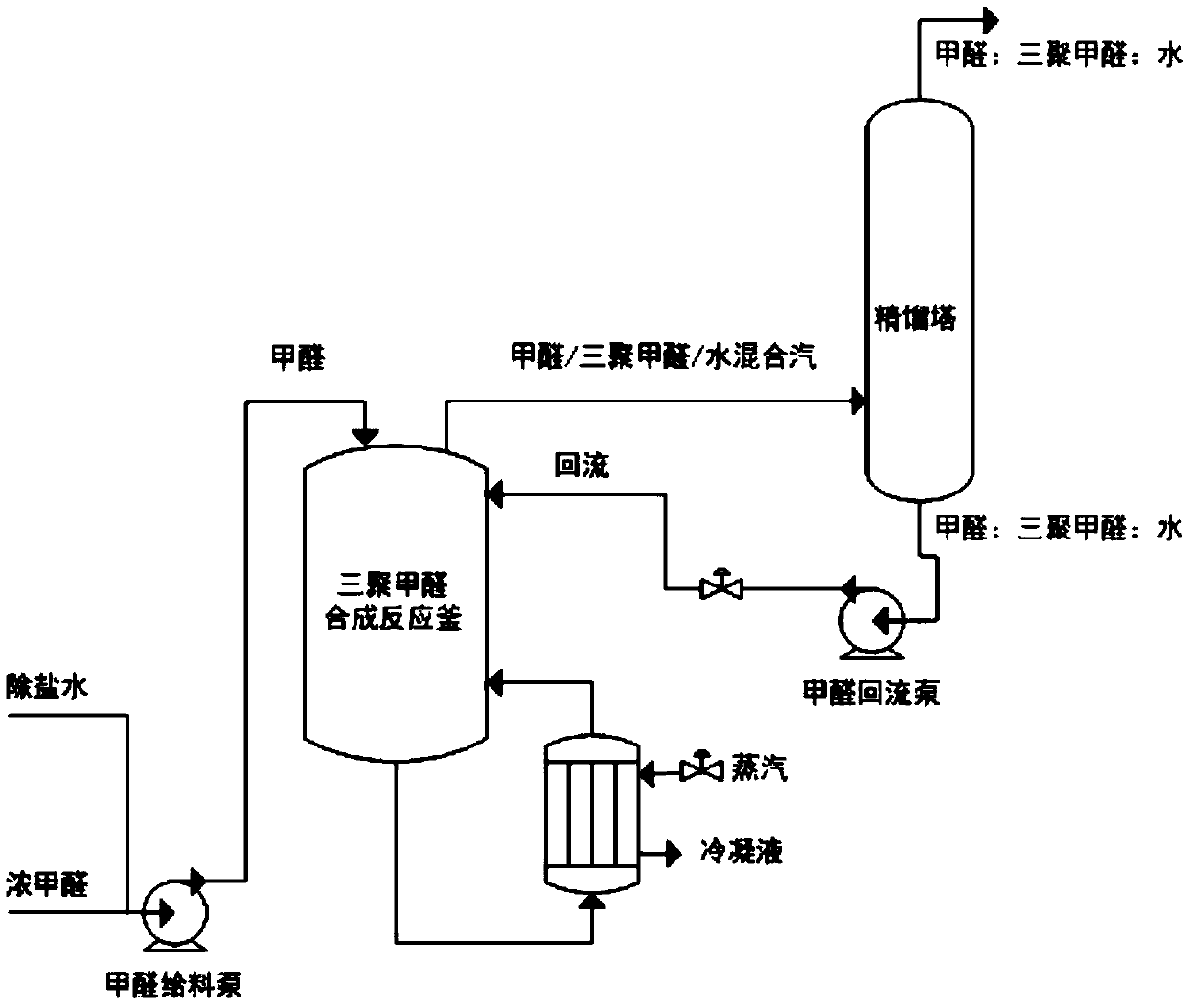
[0056] Such as figure 1 Shown, the on-line depolymerization method of by-product paraformaldehyde in a kind of trioxane synthesis reaction comprises the steps:
[0057] (1) Increase the concentration of sulfuric acid in the above-mentioned reaction solution to 6wt%, and calculate the amount of fresh sulfuric acid added according to the total amount of the reaction kettle liquid. It is necessary to exchange heat and transfer heat with circulating water through a graphite heat exchanger, store it in a dilute acid storage tank after mixing, and transport it to a paraformaldehyde synthesis...
Embodiment 2
[0063] The paraformaldehyde synthesis reaction solution to be depolymerized: the main components are 62.1wt% formaldehyde and 7.2wt% paraformaldehyde, the reaction temperature is 120°C, the catalyst sulfuric acid concentration is 4.55wt%, the heating steam is 0.3MPa, and the reaction kettle liquid is taken to stand The volume content of the by-product paraformaldehyde precipitation is 75 Vol%.
[0064] Such as figure 1 Shown, the on-line depolymerization method of by-product paraformaldehyde in a kind of trioxane synthesis reaction comprises the steps:
[0065] (1) the sulfuric acid concentration in the above-mentioned reaction solution is brought up to 8wt%, and method is with embodiment 1;
[0066] (2) After adding sulfuric acid, continuously add desalted water to the reaction solution to dilute the formaldehyde concentration, and control the flow rate of desalted water at 5m 3 Between / h, at the same time, the amount of 0.3MPa steam added to the reactor reboiler is increa...
Embodiment 3
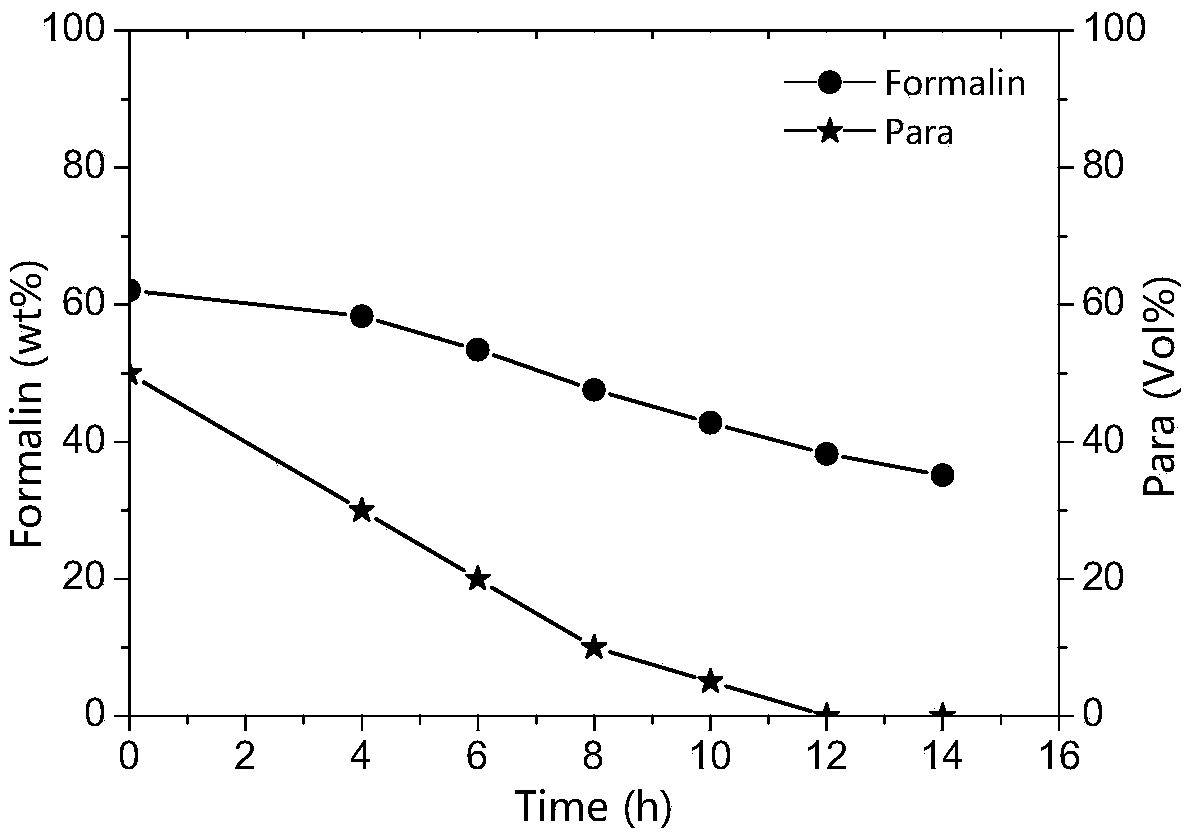
[0071] The paraformaldehyde synthesis reaction liquid to be depolymerized: the main components are 63.5wt% formaldehyde and 6.5wt% paraformaldehyde, the reaction temperature is 115°C, the catalyst p-toluenesulfonic acid concentration is 4.95wt%, the heating steam is 0.3MPa, and the The precipitated paraformaldehyde content of the product was 70 Vol%.
[0072] Such as figure 1 Shown, the on-line depolymerization method of by-product paraformaldehyde in a kind of trioxane synthesis reaction comprises the steps:
[0073] (1) The p-toluenesulfonic acid concentration in the above-mentioned reaction solution is increased to 7.51wt%, and the method is the same as in Example 1;
[0074] (2) After adding p-toluenesulfonic acid, continuously add desalted water to the reaction solution to dilute the formaldehyde concentration, and control the flow rate of desalted water at 5m 3 Between / h, at the same time, the amount of 0.3MPa steam added to the reactor reboiler is increased by 5t / h, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com