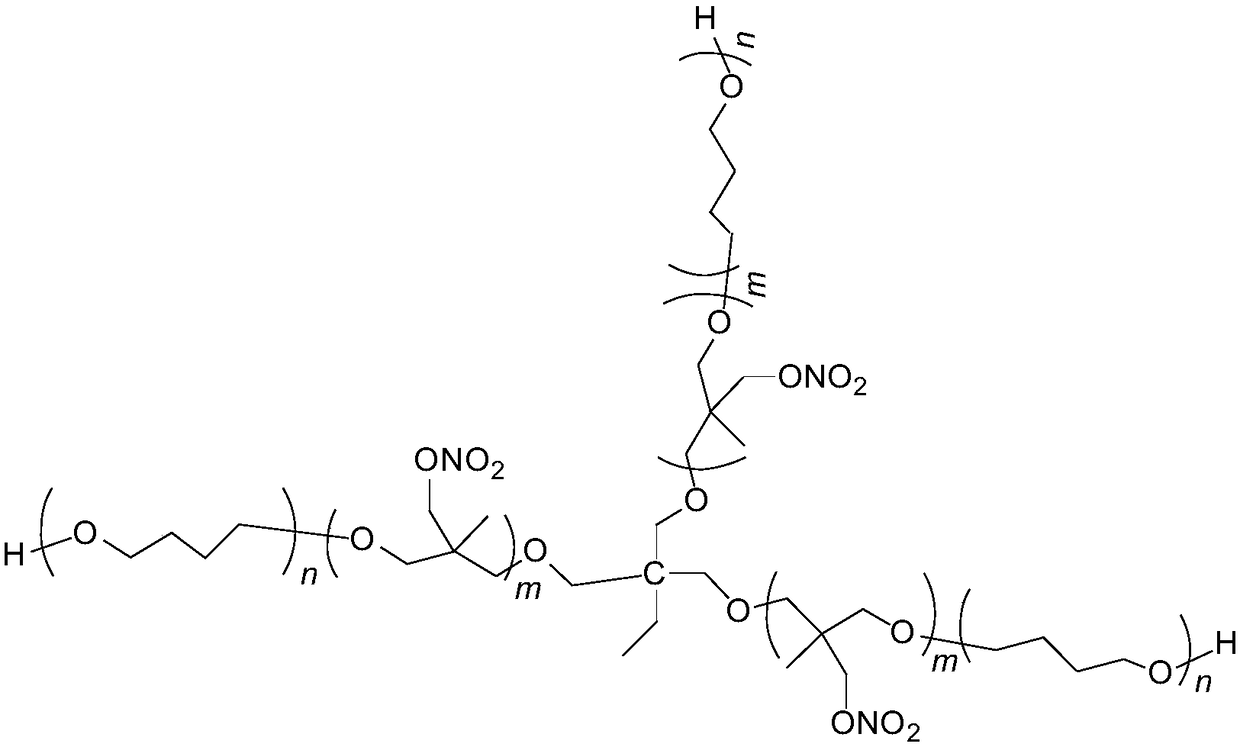
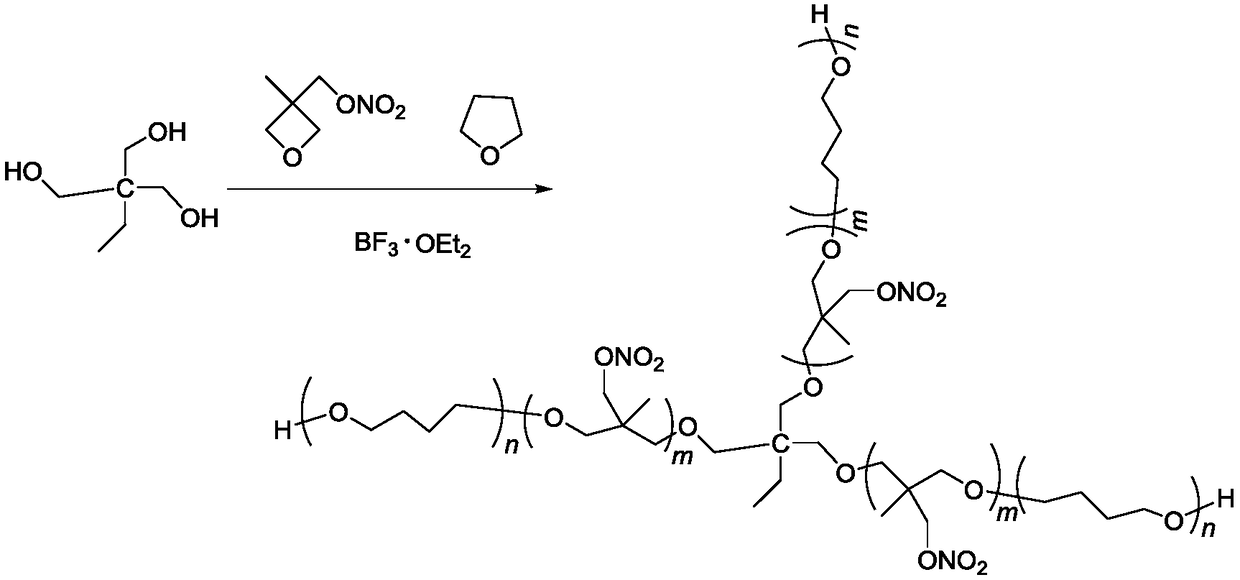
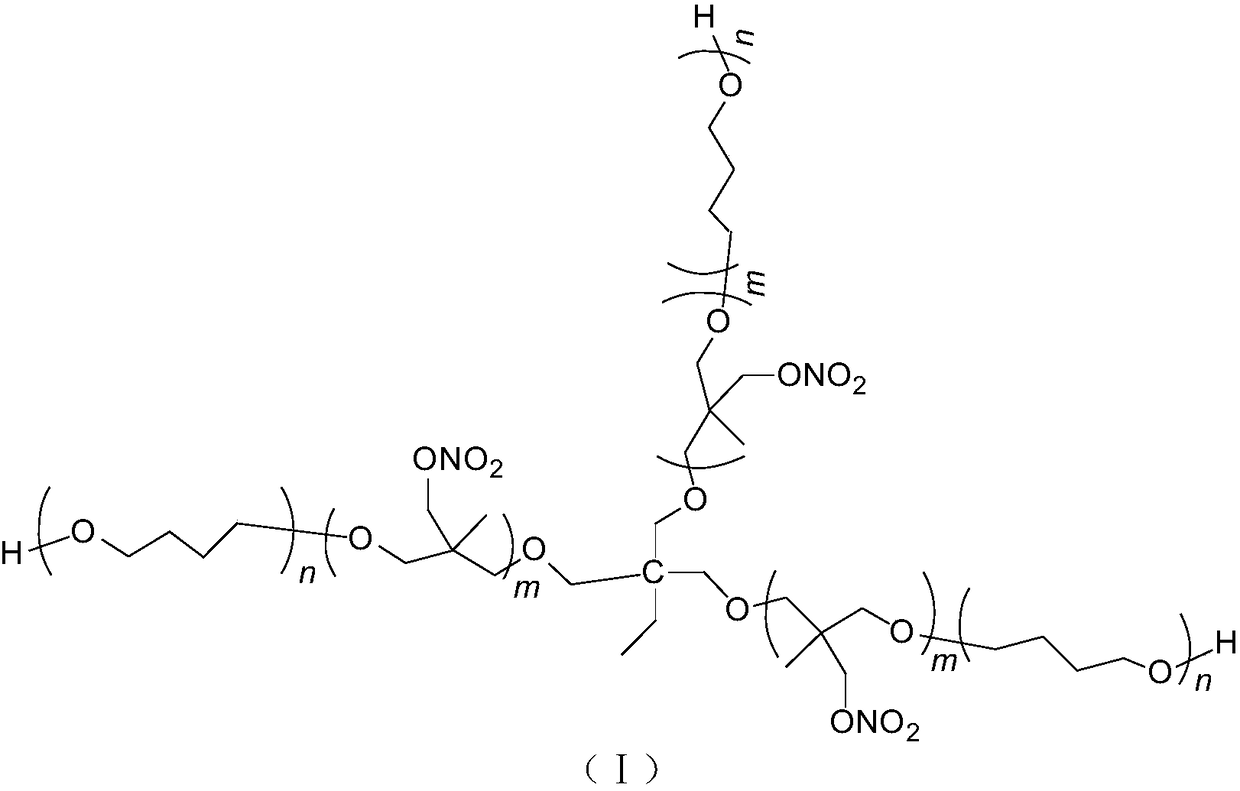
Tri-functionality NIMMO-THF (3-nitatomcthyl-3-methyl oxetane-tetrahydrofuran) copolymer ether energetic binder and synthetic method thereof
A technology with three functionalities and synthetic methods, applied in the direction of polyurea/polyurethane adhesives, adhesive types, adhesives, etc., can solve problems such as not easy to stir evenly, high viscosity of the system, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 0.65g trimethylolpropane, 5.4g tetrahydrofuran, 2.1g boron trifluoride etherate complex in a 100mL four-necked round bottom flask equipped with mechanical stirring, reflux condenser, thermometer and dropping funnel, Stir for 30min, slowly add 11g of NIMMO monomer dropwise at room temperature, the dropwise addition time is 2h, and stir at room temperature for 24h after dropping. Add 50 mL of dichloromethane to dissolve, stop the reaction with saturated aqueous sodium carbonate solution, and wash with pure water three times. The organic phase was separated and the dichloromethane removed under reduced pressure to give 15.17 g of a viscous liquid.
[0022] Structure Identification:
[0023] IR, ν max (cm -1 ): 3453(-OH), 2940(-CH 3 ), 2865 (-CH 2 -), 1111 (fatty ether C-O-C), 1632, 1280, 867 (-ONO 2 ).
[0024] 1 H NMR (CDCl 3 ,500MHz): δ4.39~4.41(-CH 2 ONO 2 ),3.25~3.42(-CH 2 -O-CH 2 -),1.58~1.62(-CH 2 -CH 2 -),0.96~1.00(-CH 3 );
[0025] 13 C NMR (C...
Embodiment 2
[0029] Add 1.3g of trimethylolpropane, 5.4g of tetrahydrofuran, and 2.1g of boron trifluoride etherate complex in a 100mL four-necked round-bottomed flask equipped with mechanical stirring, reflux condenser, thermometer, and dropping funnel. Stir for 30min, slowly add 11g of NIMMO monomer dropwise at room temperature, the dropwise addition time is 2h, and stir at room temperature for 12h after dropping. Add 50 mL of dichloromethane to dissolve, stop the reaction with saturated aqueous sodium carbonate solution, and wash with pure water three times. The organic phase was separated, and the dichloromethane was removed under reduced pressure to obtain 15.75 g of a viscous liquid, which was the trifunctional NIMMO-THF copolyether energetic binder.
Embodiment 3
[0031] Add 0.65g trimethylolpropane, 5.4g tetrahydrofuran, 2.1g boron trifluoride etherate complex in a 100mL four-necked round bottom flask equipped with mechanical stirring, reflux condenser, thermometer and dropping funnel, Stir for 30 minutes, slowly add 22 g of NIMMO monomer dropwise at room temperature, the dropwise addition time is 4 hours, and stir for 24 hours at room temperature after the drop is completed. Add 50 mL of dichloromethane to dissolve, stop the reaction with saturated aqueous sodium carbonate solution, and wash with pure water three times. The organic phase was separated, and the dichloromethane was removed under reduced pressure to obtain 25.10 g of a viscous liquid, which was the trifunctional NIMMO-THF copolyether energetic binder.
[0032] Application properties of trifunctionality NIMMO-THF copolyether energetic adhesive of the present invention
[0033] 1) Thermal stability of trifunctional NIMMO-THF copolyether energetic adhesives
[0034] The t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com