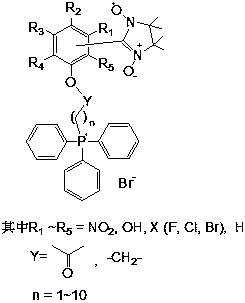
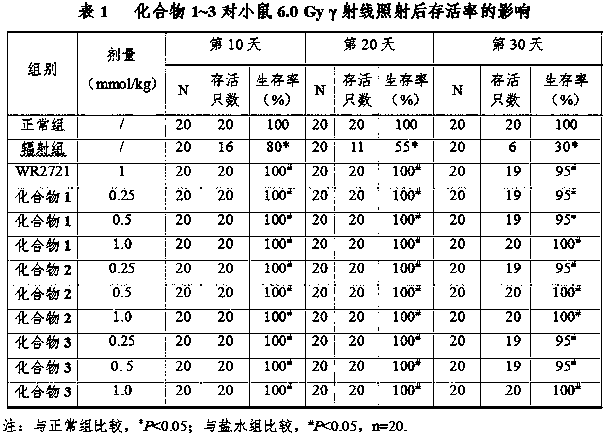
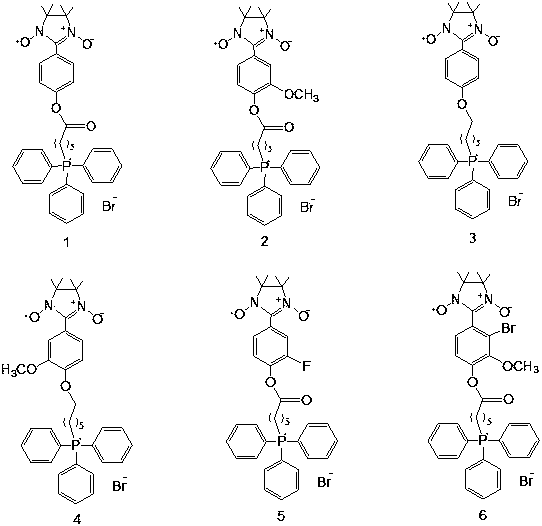Preparation of mitochondrion-targeting radioprotectant and application of mitochondrion-targeted radioprotectant in radiation damage protection
An anti-radiation and tablet technology, applied in the field of medicine, can solve the problems of severe side effects, high cytotoxicity, and short half-life, and achieve the effect of preventing cell apoptosis, obvious protective effect, and realizing protective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Synthesis method of compound 1
[0021] (1) Synthesis of p-hydroxybenzaldehyde nitroxide radical
[0022] Weigh 0.275g (2.25mol) of p-hydroxybenzaldehyde and 0.50g of 2,3-dimethyl-2,3-dihydroxyaminobutane, dissolve in 30mL methanol, stir, reflux at 78°C for 24h, spin dry, and obtain the solid Add to 30mL CH 2 Cl 2 Medium, stirring, 0℃, add saturated NaIO 4 Until the solution is dark blue. Let stand, separate into layers, take the organic phase, spin-dry to obtain the crude product. TLC detection, column chromatography to obtain pure product.
[0023] (2) Synthesis of 5-carboxypentyl phosphine bromide
[0024] Weigh 2.62g (TPP, 10mmol) of triphenylphosphine and 2.07g (10.5mmol) of 6-bromohexanoic acid, dissolve in anhydrous acetonitrile, protect under nitrogen, and react under reflux for 16h. The pure product is obtained after recrystallization.
[0025] (3) Synthesis of compound 1
[0026] Weigh 0.199g (0.8mmol) of p-hydroxybenzaldehyde nitroxide radical, 0.469g (1....
Embodiment 2
[0027] Example 2: Synthesis method of compound 2
[0028] (1) Synthesis of vanillin nitroxide radical
[0029] Weigh 0.342g (2.25mol) of vanillin and 0.50g of 2,3-dimethyl-2,3-dihydroxylaminobutane, dissolve in 30mL methanol, stir, reflux at 78°C for 24h, spin dry, and add the resulting solid to 30mL CH 2 Cl 2 Medium, stirring, 0℃, add saturated NaIO 4 Until the solution is dark blue. Let stand, separate into layers, take the organic phase, spin-dry to obtain the crude product. TLC detection, column chromatography to obtain pure product.
[0030] (2) Synthesis of 5-carboxypentyl phosphine bromide
[0031] Weigh 2.62g (TPP, 10mmol) of triphenylphosphine and 2.07g (10.5mmol) of 6-bromohexanoic acid, dissolve in anhydrous acetonitrile, protect under nitrogen, and react under reflux for 16h. The pure product is obtained after recrystallization.
[0032] (3) Synthesis of compound 2
[0033] Weigh 0.237g (0.8mmol) of vanillin nitroxide radical, 0.469g (1.2mmol) of 5-carboxypentylphosphoniu...
Embodiment 3
[0034] Example 3: Synthesis method of compound 3
[0035] (1) Synthesis of p-hydroxybenzaldehyde nitroxide radical
[0036] Weigh 0.275g (2.25mol) of p-hydroxybenzaldehyde and 0.50g of 2,3-dimethyl-2,3-dihydroxyaminobutane, dissolve in 30mL methanol, stir, reflux at 78°C for 24h, spin dry, and obtain the solid Add to 30mL CH 2 Cl 2 Medium, stirring, 0℃, add saturated NaIO 4 Until the solution is dark blue. Let stand, separate into layers, take the organic phase, spin-dry to obtain the crude product. TLC detection, column chromatography to obtain pure product.
[0037] (2) Synthesis of 6-bromohexylphosphonium bromide
[0038] Weigh 2.62g (TPP, 10mmol) of triphenylphosphine and 7.3g (30mmol) of 1,6-dibromohexane, dissolve in anhydrous acetonitrile, and reflux for 12h. TLC detection, column chromatography to obtain pure product.
[0039] (3) Synthesis of compound 3
[0040] Weigh 0.199g (0.8mmol) of p-hydroxybenzaldehyde nitroxide radical, 0.51g (1mmol) of 6-bromohexyl phosphine bromide...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com