Recovery method for byproduct, p-chlorobenzoic acid, during production of p-chlorobenzaldehyde
A technology of p-chlorobenzaldehyde and p-chlorobenzoic acid, which is applied in chemical instruments and methods, preparation of organic compounds, separation/purification of carboxylic acid compounds, etc., can solve the problem of reducing the purity of p-chlorobenzoic acid, increasing industrial costs, and unfavorable Reuse and other issues to achieve the effect of low recycling cost, good quality and simple recycling process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
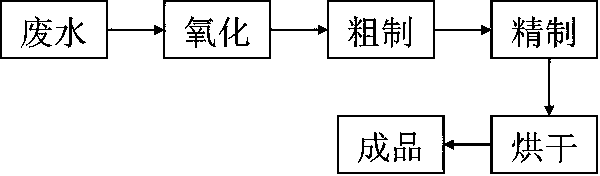
[0028] Pump 5000 L of washing water from the production of p-chlorobenzaldehyde into the enamel reaction kettle, add 10 kg of potassium permanganate, raise the temperature to 60 ± 5 °C for 2 h, and let it settle for 1 h to remove the mechanical impurities and Organic insoluble matter; use negative pressure to extract 3200 L of the solution into another enamel reaction kettle, add 640 kg of toluene, and add 290 L of industrial hydrochloric acid (31%) dropwise. At this time, the pH of the solution is 6 ~ 7, and stir well 1 ~ 1.5h, all the p-chlorobenzoic acid in the solution is precipitated, and when the temperature rises to 70 ± 5 ℃, the p-chlorobenzoic acid is completely dissolved in toluene, and the layers are separated after standing, and the liquid in the water layer is transported to the waste water tank, and then sent to the sewage treatment Station, the organic layer was cooled to normal temperature; the organic layer was cooled with refrigerated brine and started to stir...
Embodiment 2
[0030] Pump 5000 L of washing water from the production of p-chlorobenzaldehyde into an enamel reaction kettle, add 15 kg of hydrogen peroxide, heat up to 60±5°C for 2 h of oxidation, let it settle for 1 h, and remove mechanical impurities and organic insolubles in the lower layer ; Extract 3200 L of the solution in another enamel reaction kettle with negative pressure, add 640 kg of toluene, and add 290 L of industrial hydrochloric acid (31%) dropwise. After 1.5 hours, all the p-chlorobenzoic acid in the solution was precipitated, and when the temperature was raised to 70±5°C, the p-chlorobenzoic acid was completely dissolved in toluene, allowed to stand for stratification, and the liquid in the water layer was transported to the waste water tank, and then sent to the sewage treatment station. Cool the organic layer to room temperature; cool the organic layer with refrigerated brine and start stirring, and continue stirring for 1 h when the temperature drops to 0 °C, all p-chl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com