An alpha-amylase mutant with improved enzymatic activity and applications thereof
A technology of amylase and mutants, which is applied in the field of genetic engineering and microbial engineering, can solve the problems of unfavorable human body, difficult and limited application of α-amylase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Preparation of α-amylase mutants
[0051] (1) Preparation of α-amylase single mutant
[0052] According to the gene sequence of α-amylase whose amino acid sequence is shown in SEQ ID NO.1, primers for introducing K82E and S405R mutations were designed and synthesized, and rapid PCR technology was used to carry the recombination of the gene encoding wild-type α-amylase The vector pET20b-amyS template was used to perform site-directed mutation on the α-amylase gene, and the DNA coding sequence was determined. It was identified that the 82nd Lys codon was changed into a Glu codon, and the 405th Ser codon was changed into an Arg codon. single mutant alpha-amylase;
[0053] Among them, the recombinant vector pET20b-amyS was preserved in the laboratory (Ref: L Z, D X, W J. Improving thethermostability and enhancing the Ca 2+ binding of the maltohexaose-forming α-amylase from Bacillus stearothermophilus. Journal of Biotechnology. 2016; 222:65–72.).
[0054] The s...
Embodiment 2
[0072] Embodiment 2: α-amylase mutant shakes flask fermentation to produce enzyme verification in Escherichia coli
[0073] (1) Shake flask fermentation of α-amylase mutants in Escherichia coli to produce enzymes
[0074] Pick recombinant bacteria E.coli BL21(DE3) / pET20b-amyS(K82E), E.coli BL21(DE3) / pET20b-amyS(S405), E.coli BL21(DE3) / pET20b-amyS(K82E / S405R ) at 37°C in LB liquid medium (containing 100 μg / mL ampicillin) for 8 to 10 hours, and the seed fermentation liquid was connected to the fermentation medium (containing 100 μg / mL ampicillin) according to the inoculum size of 5%, at 25 After culturing in a shaking table at ℃ for 48 hours, the fermentation broth was centrifuged at 4 ℃ and 8000 rpm for 10 minutes to remove bacteria, and the centrifuged supernatant was collected to obtain a crude enzyme solution.
[0075] (2) Determination of enzyme activity of α-amylase mutants in Escherichia coli shake flask fermentation
[0076] Determination of α-amylase mutants K82E, S40...
Embodiment 3
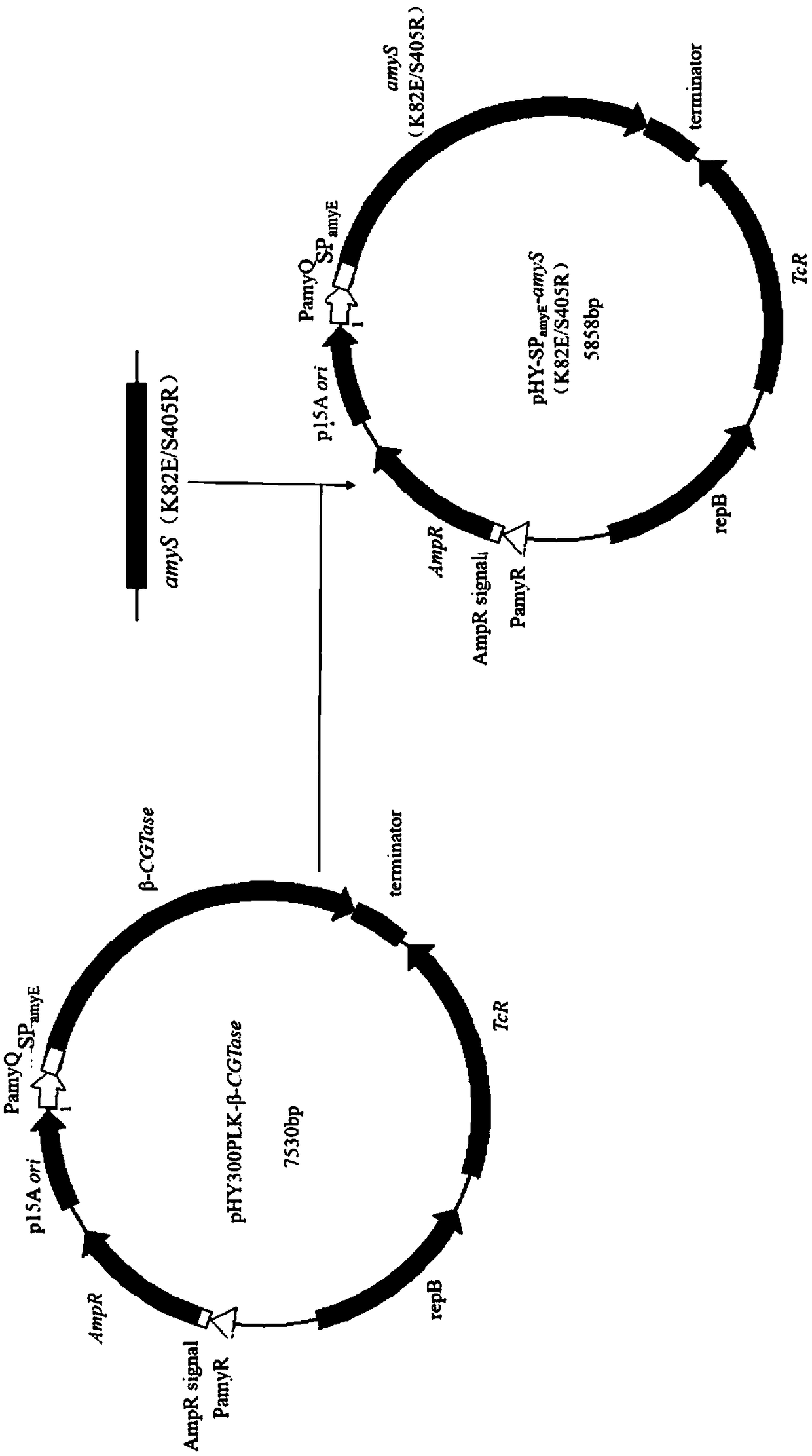
[0079] Example 3: Construction of recombinant vector pHY-SP amyE -amyS, pHY-SP amyE -amyS(K82E / S405R)
[0080] The specific steps are as follows (the construction process see figure 1 ):
[0081] (1) Design primers amyS-F and amyS-R containing homology arms, use recombinant vectors pET20b-amyS and pET20b-amyS (K82E / S405R) as templates, and PCR amplify amyS and amyS with homology arms ( K82E / S405R)
[0082] (2) Design primers pHY-F and pHY-R, and use the recombinant vector pHY300PLK-β-CGTase as a template to amplify the vector pHY-SP by PCR amyE , wherein the recombinant vector pHY300PLK-β-CGTase is preserved in the laboratory (Ref: Zhang, K., Duan, X., Wu, J. 2016. Multigene disruption in undomesticated Bacillus subtilis ATCC 6051 ausing the CRISPR / Cas9 system. Scientific Reports, 6. Bacillus subtilis).
[0083] The primer sequences are shown in Table 3:
[0084] Table 3 Primer Sequence
[0085]
[0086]
[0087] Note: The underlined part is the homology arm sequ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com