Medical modified collagen matrix drug loading sponge and preparation method thereof
A collagen matrix and matrix technology, applied in the field of modified collagen matrix drug-loaded medical sponge and its preparation, can solve the problems of small loading dose and insignificant drug effect, and achieve large drug-loading dose, significant drug effect, and bonding Effects with just the right intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
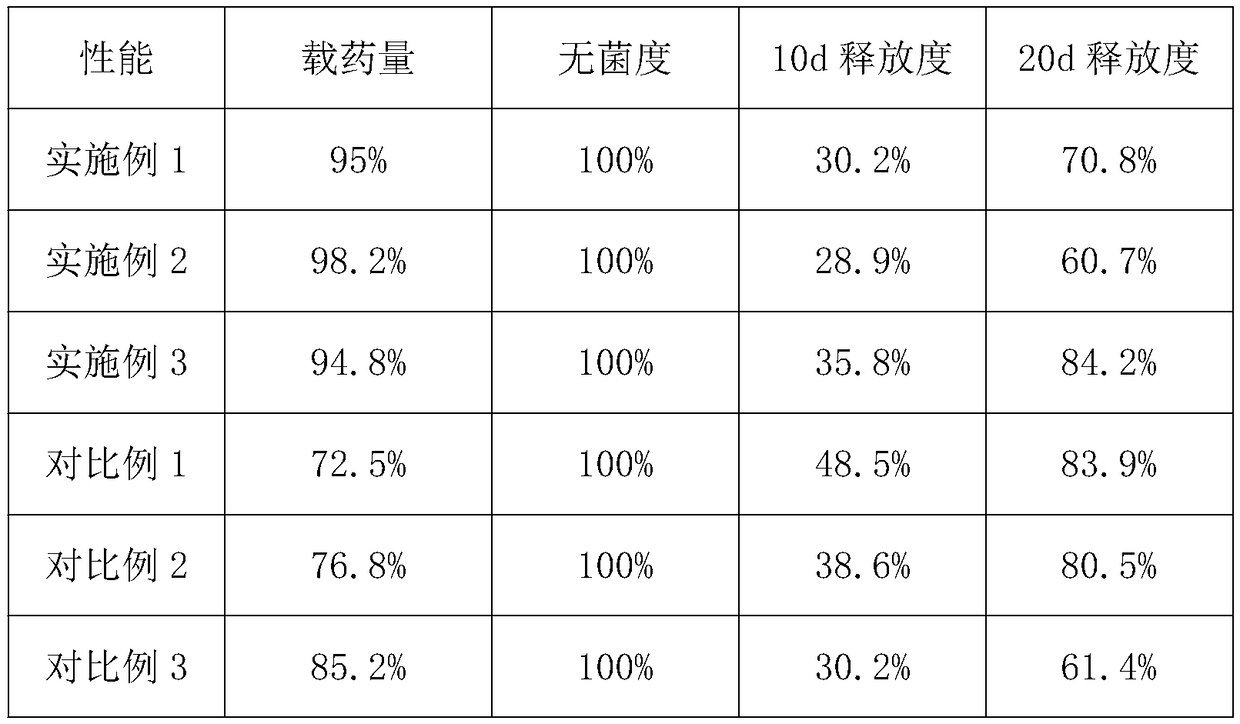
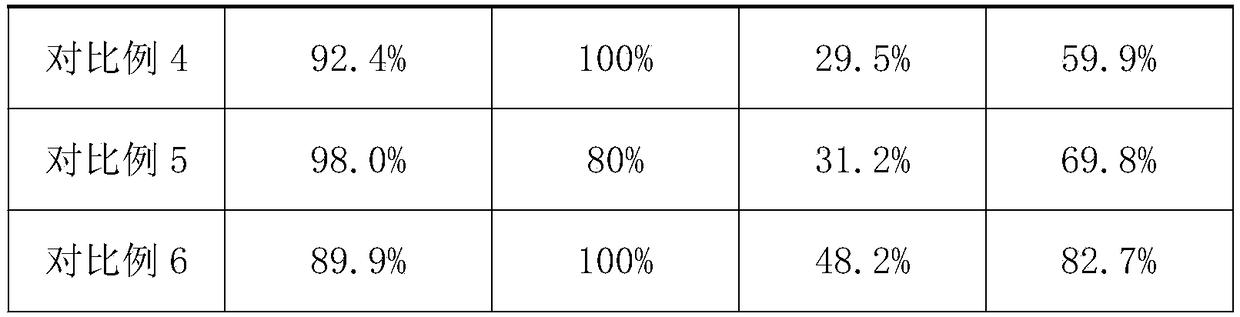
Embodiment 1
[0032](1) With microbial modifier, press microbial modifier 15%, peptone 2%, agar 0.5% and water 80% after mixing, after cultivating at 20 ℃ for 2h, obtain modified finishing liquid; Described microbial modifier The mass ratio of cellulobacterium fusarium, Acetobacter xylinum and yeast is 1:1:3; the drug carrier liquid is prepared by mixing the bletilla striata polysaccharide drug carrier with a solid-liquid ratio of 2:1 and pure water;
[0033] (2) taking the collagen solution with a mass fraction of 20%, and respectively irradiating the pores with ultraviolet light to make a porous collagen matrix with a porosity of 80%;
[0034] (3) Immerse the porous collagen matrix prepared in step (2) into the modified finishing solution prepared in step (1), and perform modification treatment at 30° C. for 20 h.
[0035] (4) Take out the modified porous collagen matrix in the step (3), at 60Kw.s / cm 2 Under the ultraviolet irradiation intensity, the irradiation time is 20h, carry out ul...
Embodiment 2
[0038] (1) Use microbial modifier, press microbial modifier 30%, peptone 3%, after agar 2% and water 60% mix, obtain modified finishing solution after cultivating at 40 ℃ for 1h; Described microbial modifier The mass ratio of cellulobacterium fusarium, Acetobacter xylinum and yeast is 1:3:5; chitosan drug carrier is mixed with pure water at a solid-liquid ratio of 4:1 to prepare a drug carrier liquid;
[0039] (2) Take the collagen solution with a mass fraction of 40%, and use the physical cross-linking pore-forming method to prepare a porous collagen matrix with a porosity of 60%;
[0040] (3) Immerse the porous collagen matrix prepared in step (2) in the modified finishing solution prepared in step (1), and perform modification treatment at 50° C. for 16 hours.
[0041] (4) Take out the modified porous collagen matrix in the step (3), at 20Kw.s / cm 2 Under the intensity of ultraviolet irradiation, the irradiation time is 40h, ultraviolet sterilization is carried out to kill ...
Embodiment 3
[0044] (1) With microbial modifier, after mixing 20% of microbial modifier, 2.5% of peptone, 1% of agar and 70% of water, after cultivating at 30°C for 1.5h, a modified finishing solution is obtained; The mass ratio of cellulobacterium fusarium, Acetobacter xylinum and saccharomyces in the agent is 1:2:4; the drug carrier liquid is prepared by mixing the cartin hydrochloride drug carrier with pure water at a solid-liquid ratio of 5:1;
[0045] (2) Take the collagen solution with a mass fraction of 30%, and use chemical cross-linking pore-forming methods to prepare porous collagen matrices with a porosity of 65%;
[0046] (3) Immerse the porous collagen matrix prepared in step (2) into the modified finishing solution prepared in step (1), and perform modification treatment at 60° C. for 5 hours.
[0047] (4) Take out the modified porous collagen matrix in the step (3), at 40Kw.s / cm 2 Under the intensity of ultraviolet irradiation, the irradiation time is 30h, ultraviolet ste...
PUM
| Property | Measurement | Unit |
|---|---|---|
| porosity | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com