Method for hydrogen transfer reduction of nitrogenous heterocyclic compounds
A nitrogen heterocyclic compound and hydrogen transfer technology, applied in organic chemistry and other fields, can solve the problems of expensive raw materials and achieve good reproducibility, high yield, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
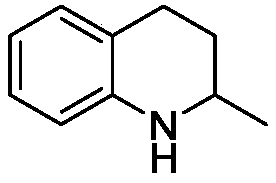
[0025] Embodiment 1: the preparation of 2-methyl-1,2,3,4-tetrahydroquinoline
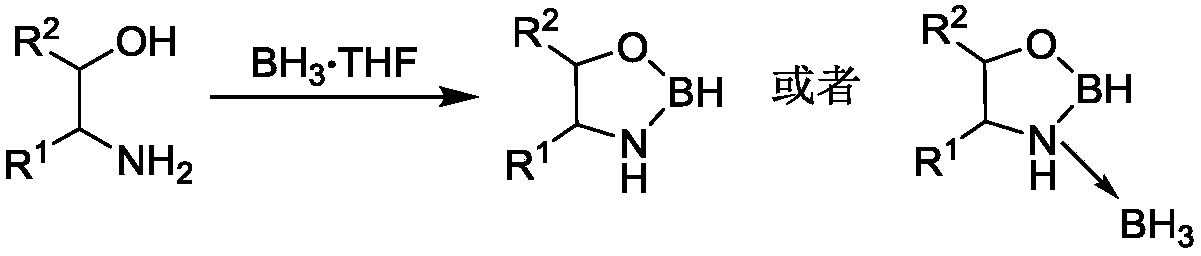
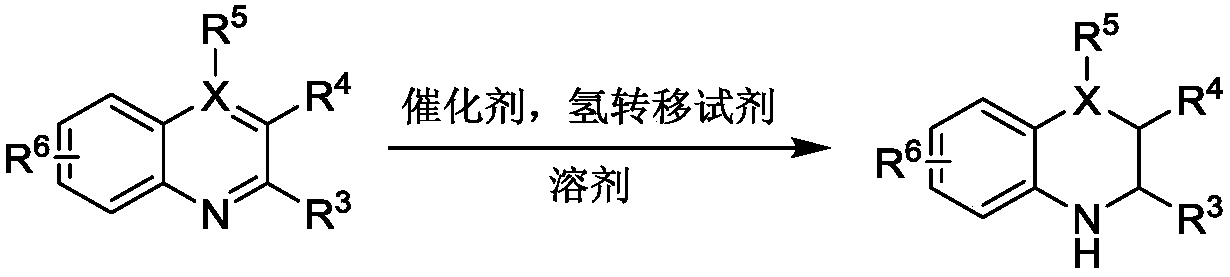
[0026] Weigh ethanolamine (61.08mg, 1.0mmol) into a Shrek tube, under the protection of an inert gas, cool to 0°C, add borane tetrahydrofuran complex (1M, 2.0mmol), stir at room temperature for 24h, vacuum the solvent The obtained oxazoboridine was directly used in the next step. Add 2-methylquinoline (71.5 mg, 0.5 mmol), tetrahydrofuran (2.0 mL), copper perchlorate hexahydrate (37.05 mg, 0.2 equiv). The reaction was stirred at room temperature for 24h. The obtained reactant was purified by silica gel column (petroleum ether / ethyl acetate=10:1) to obtain 2-methyl-1,2,3,4-tetrahydroquinoline (69.0 mg) with a yield of 93.9%.
[0027]
[0028] 2-Methyl-1,2,3,4-tetrahydroquinoline
[0029] 1 H NMR (400MHz, CDCl 3 )δ7.03-6.97 (m, 2H), 6.65 (td, J = 7.4Hz, 1.2Hz, 1H), 6.51 (dd, J = 8.2Hz, 1.2Hz, 1H), 3.70 (br, NH), 3.48 -3.38(m,1H),2.93-2.83(m,1H),2.81-2.73(m,1H),2.01-1.93(m,1H),1.68-1.58(m,1H),...
Embodiment 2
[0030] Embodiment 2: Preparation of 2-methyl-1,2,3,4-tetrahydroquinoline
[0031]Weigh ethanolamine (61.08mg, 1.0mmol) into a Shrek tube, under the protection of an inert gas, cool to 0°C, add borane tetrahydrofuran complex (1M, 2.0mmol), stir at room temperature for 24h, vacuum the solvent The obtained oxazoboridine was directly used in the next step. Add 2-methylquinoline (71.5 mg, 0.5 mmol), tetrahydrofuran (2.0 mL), copper trifluoromethanesulfonate (72.34 mg, 0.2 equiv). The reaction was stirred at room temperature for 24h. The obtained reactant was purified by silica gel column (petroleum ether / ethyl acetate=10:1) to obtain 2-methyl-1,2,3,4-tetrahydroquinoline (61.45 mg) with a yield of 83.5%.
[0032]
[0033] 2-Methyl-1,2,3,4-tetrahydroquinoline
[0034] 1 H NMR (400MHz, CDCl 3 )δ7.03-6.97 (m, 2H), 6.65 (td, J = 7.4Hz, 1.2Hz, 1H), 6.51 (dd, J = 8.2Hz, 1.2Hz, 1H), 3.70 (br, NH), 3.48 -3.38(m,1H),2.93-2.83(m,1H),2.81-2.73(m,1H),2.01-1.93(m,1H),1.68-1.58(m,1H),1....
Embodiment 3
[0035] Embodiment 3: Preparation of 2-methyl-1,2,3,4-tetrahydroquinoline
[0036] Weigh ethanolamine (61.08mg, 1.0mmol) into a Shrek tube, under the protection of an inert gas, cool to 0°C, add borane tetrahydrofuran complex (1M, 2.0mmol), stir at room temperature for 24h, vacuum the solvent The obtained oxazoboridine was directly used in the next step. Add 2-methylquinoline (71.5 mg, 0.5 mmol), methanol (2.0 mL), copper perchlorate hexahydrate (37.05 mg, 0.2 equiv). The reaction was stirred at room temperature for 24h. The obtained reactant was purified by silica gel column (petroleum ether / ethyl acetate=10:1) to obtain 2-methyl-1,2,3,4-tetrahydroquinoline (48.5mg) with a yield of 65.9%.
[0037]
[0038] 2-Methyl-1,2,3,4-tetrahydroquinoline
[0039] 1 H NMR (400MHz, CDCl 3 )δ7.03-6.97 (m, 2H), 6.65 (td, J = 7.4Hz, 1.2Hz, 1H), 6.51 (dd, J = 8.2Hz, 1.2Hz, 1H), 3.70 (br, NH), 3.48 -3.38(m,1H),2.93-2.83(m,1H),2.81-2.73(m,1H),2.01-1.93(m,1H),1.68-1.58(m,1H),1.25(d,J= 6...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap