Diarylpyrimidine derivative containing biphenyl structure and preparation method and application thereof
A technology of diaryl pyrimidine and biphenyl structure, applied in the field of medicine, can solve the problem of drug loss of activity, and achieve the effects of low cytotoxicity, high selectivity coefficient, and strong biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
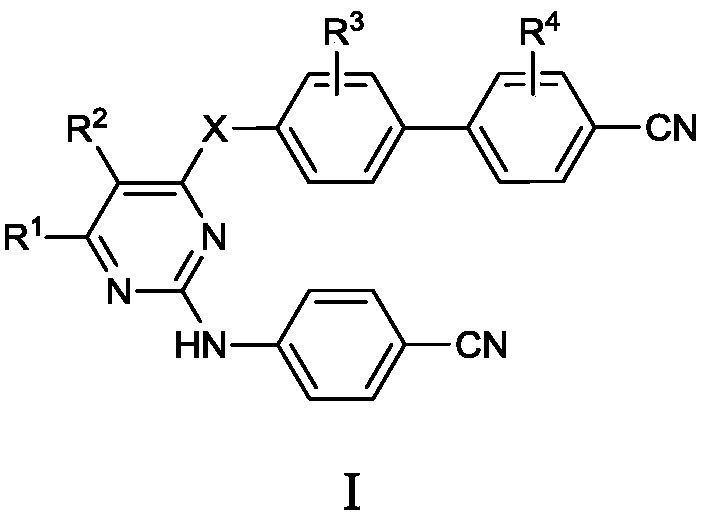
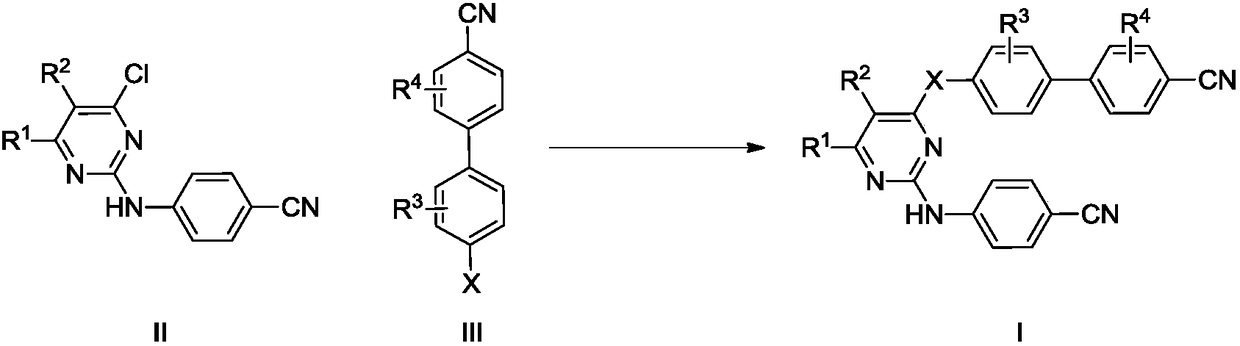
[0026] Embodiment 1: the synthesis of final product I
[0027] Add compound III and base into the solvent, stir for 30 min, then add compound II, stir at 30-100° C. for 4-10 h. The reaction was monitored by TLC until the starting material was consumed completely. The reaction solution was poured into ethyl acetate, washed with water and saturated brine successively, and the organic phase was dried over anhydrous sodium sulfate overnight. After filtration, concentration, and recrystallization from ethyl acetate and petroleum ether, the desired solid was obtained.
[0028] The target compounds were obtained by using the above methods with raw materials containing different substituents, and some results are as follows:
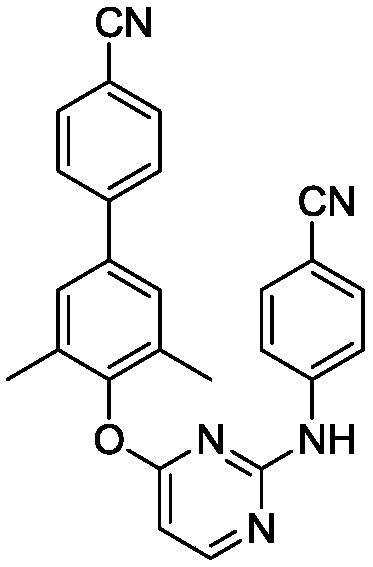
[0029] 4'-Hydroxy-3',5'-dimethyl[1,1'-biphenyl]-4-cyanocyanide (4.48mmol) and potassium carbonate (6.72mmol) were added to N,N-diphenyl Methylformamide (20 mL), stirred for 30 min, then added 2-(4-chlorophenyl)amino-4-chloropyrimidine (4.93 mmol), stirred at ...
Embodiment 2
[0058] Embodiment 2: anti-HIV biological activity test
[0059] The anti-HIV virus activity at the cell level in vitro was determined by the Rega Institute of Pharmacy at Katholleke University in Belgium, mainly including: inhibitory activity and cytotoxicity to HIV-infected MT-4 cells. The method is as follows: make the compound in HIV-infected MT-4 cells, at different time of HIV infection, use the MTT method to measure the protective effect of the drug on the cytopathy induced by HIV mutagenesis, and calculate that 50% of the cells are free from HIV-induced cytopathy half effective concentration EC 50 , the toxicity assay is carried out in parallel with the anti-HIV activity experiment, also in MT-4 cell culture, the concentration (CC 50 ), and calculate the selectivity index SI=CC 50 / EC 50 .
[0060] Materials and Methods:
[0061] The anti-HIV activity of each compound is monitored by the inhibitory effect of the drug on the cytopathic effect caused by HIV in cells....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com