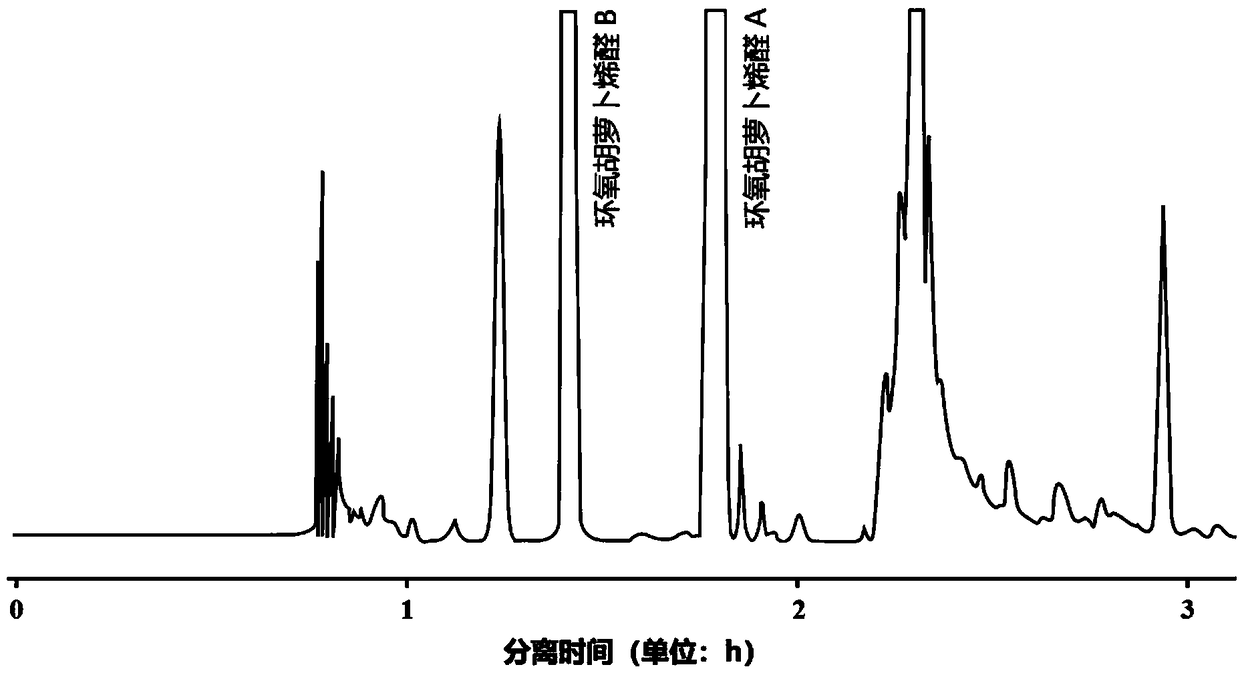
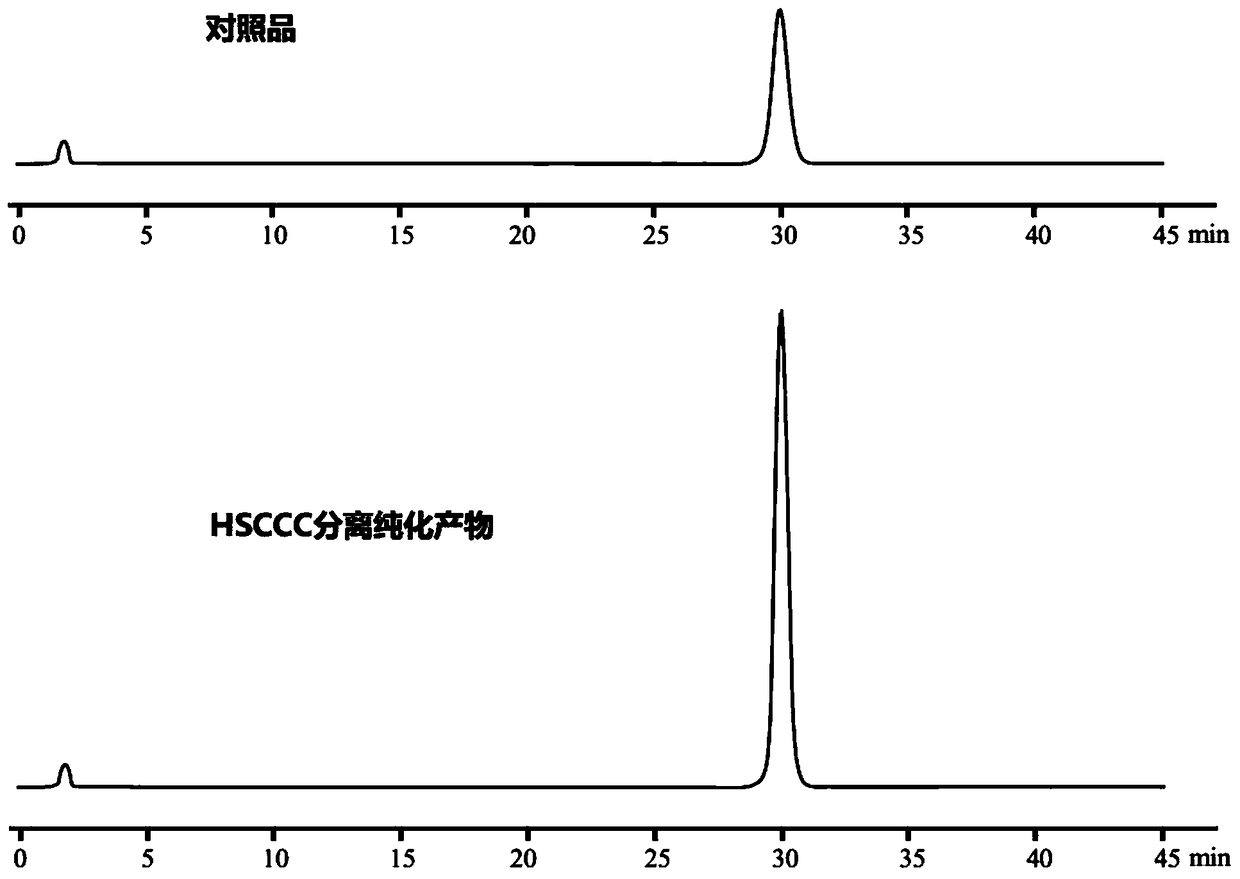
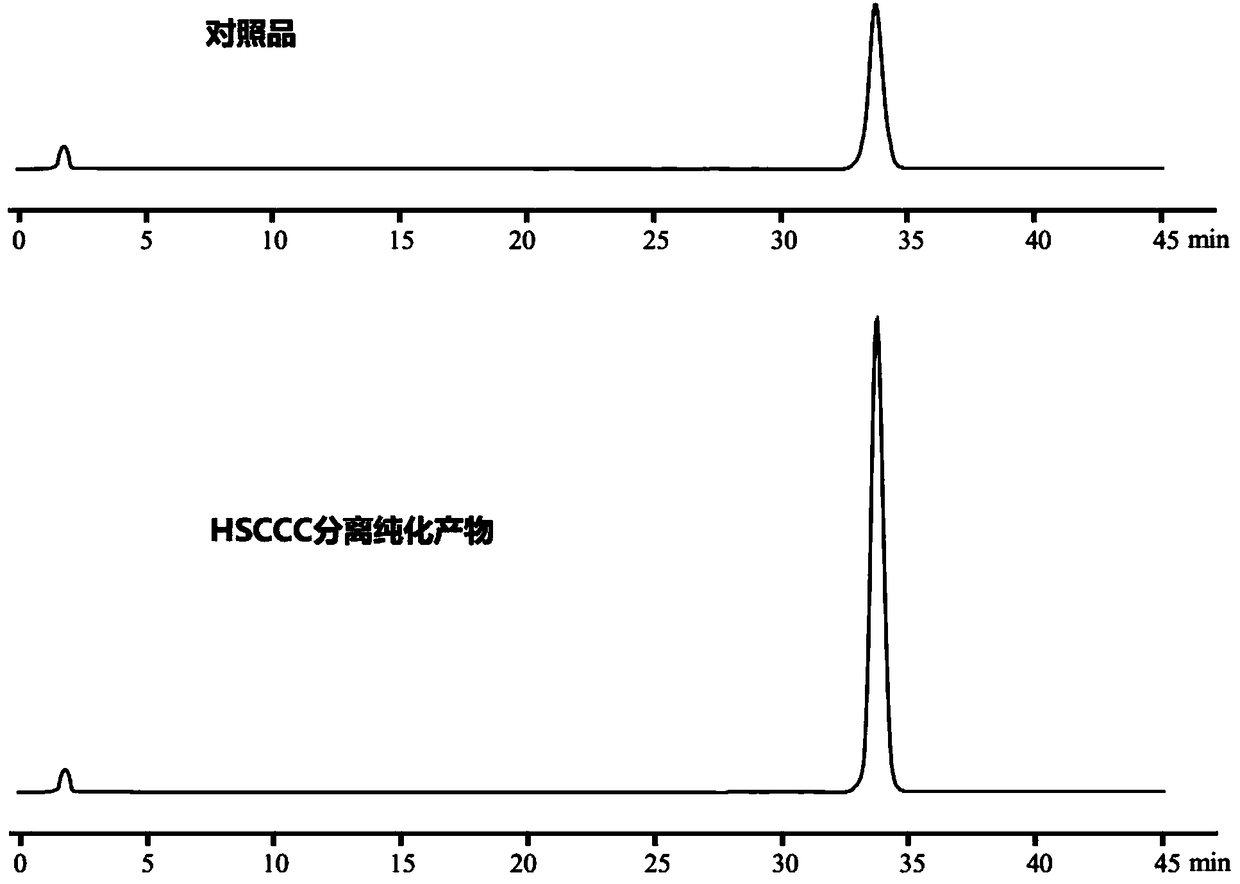
Method for preparing epoxydaucenal A and epoxydaucenal B
A technology for carrot and alkenal, applied in the field of chemistry, can solve the problems such as the lack of efficient separation and purification of epoxy carotene aldehyde, and achieve the effect of high-efficiency separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] The substantive content of the present invention will be described in detail below in conjunction with the embodiments, but the protection scope of the present invention is not limited thereto.
[0028] 1. Experimental materials
[0029] Double roses are the flowers of double roses, which are freshly picked and dried in the shade for later use.
[0030] DA-201 macroporous resin was purchased from Tianjin Haoju Resin Technology Co., Ltd.
[0031] High-speed countercurrent chromatography TEB300B was purchased from Shanghai Tongtian Biotechnology Co., Ltd.
[0032] 2. Experimental methods and results
[0033] Including the following steps:
[0034] Step S1, extraction: pulverize the double-petal roses dried in the shade, extract with 95% ethanol aqueous solution under heat reflux, the solid-to-liquid ratio is 1:20, extract 3 times, each time for 1.5h, filter, and combine the filtrates to concentrate to nothing mellow;
[0035] Step S2, macroporous resin enrichment: pu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com