Mutant protein A (Protein A) affinity chromatography medium
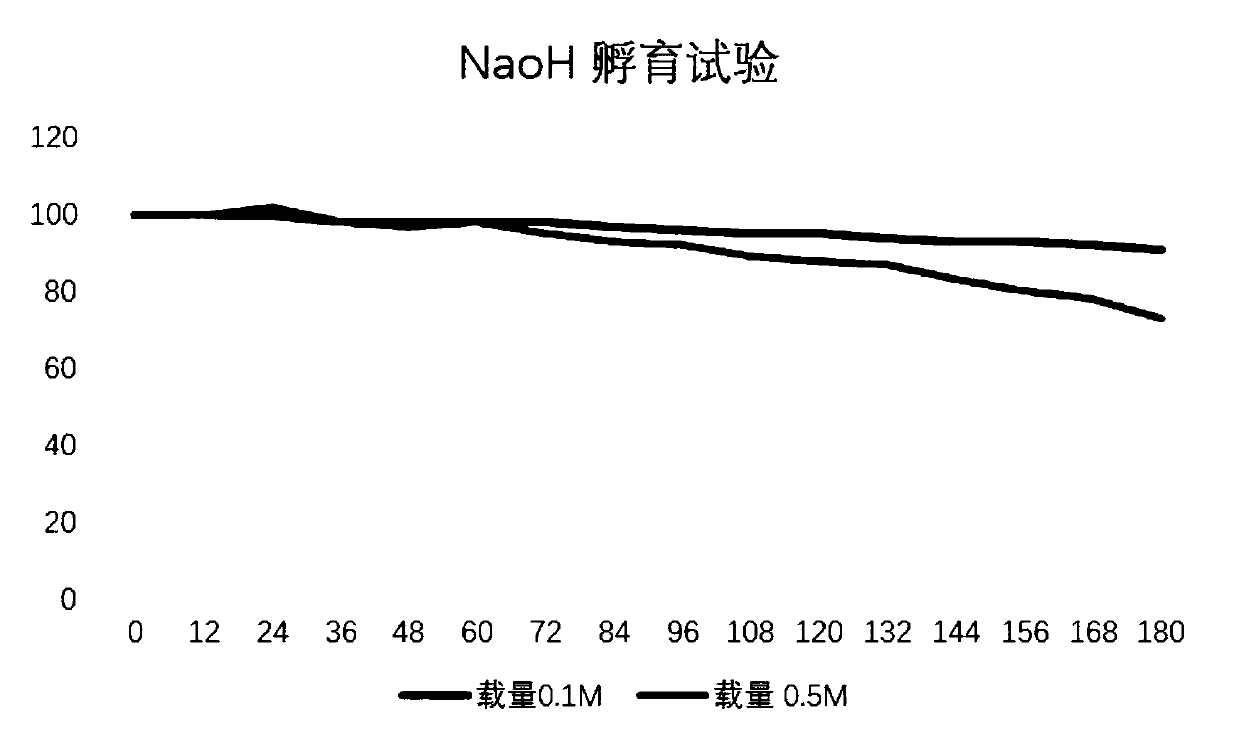
A chromatographic medium and mutant protein technology, which is applied in the field of biomedicine, can solve problems such as inability to withstand high-concentration NaOH cleaning, gaps in alkali resistance, and poor column packing repeatability, achieving good column packing repeatability, greatly improving, The effect of structural stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0023] A mutein A (Protein A) affinity chromatography medium, the mutein A (Protein A) affinity chromatography medium is used as a chromatography medium in the separation and purification of antibodies and Fc fusion proteins; the protein A ( Protein A) Affinity chromatography media are based on microspheres containing vinyl monomers, agarose or dextran gels. Suitable vinyl-containing monomers include, but are not limited to vinyl known to be used in polymerization processes Monomers, typical vinyl monomers include: styrene, methylstyrene, all isomers of vinyltoluene, and p-vinyltoluene, all isomers of ethylstyrene, propylstyrene, ethylene Naphthalene, vinylanthracene, and mixtures thereof. Vinyl monomers can also be combined with other copolymerizable monomers. Examples of such monomers include, but are not limited to, alkenonitrile and acrylate monomers, such as acrylonitrile, methacrylonitrile, acrylates, methacrylates, and mixtures thereof.
[0024] The microspheres of th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com