Synthetic method of tazobactam diphenylmethyl ester
The technology of Zobactam diphenylmethyl ester and synthesis method is applied in the field of drug synthesis, which can solve the problems of many by-products, low product yield, environmental pollution, etc., and achieve the reduction of waste solid generation, simple post-processing operation, and improved The effect of purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
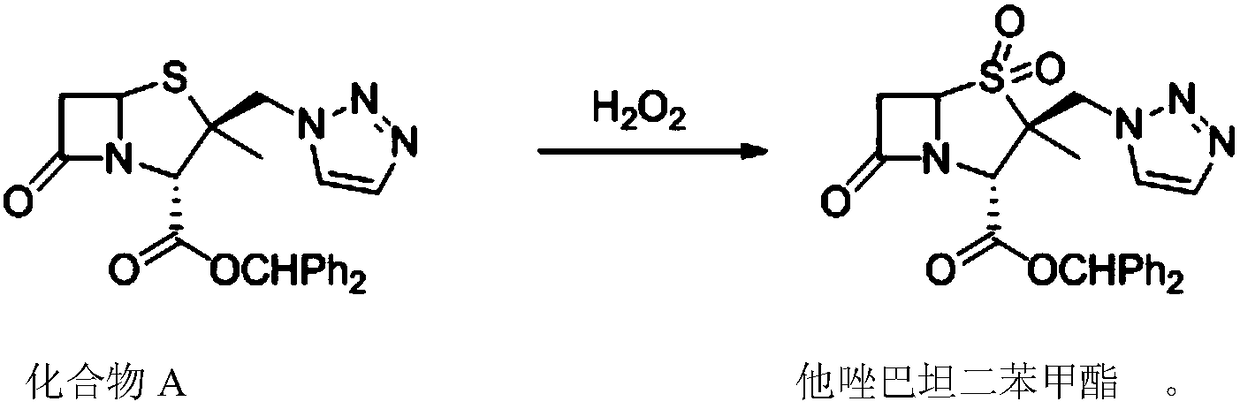
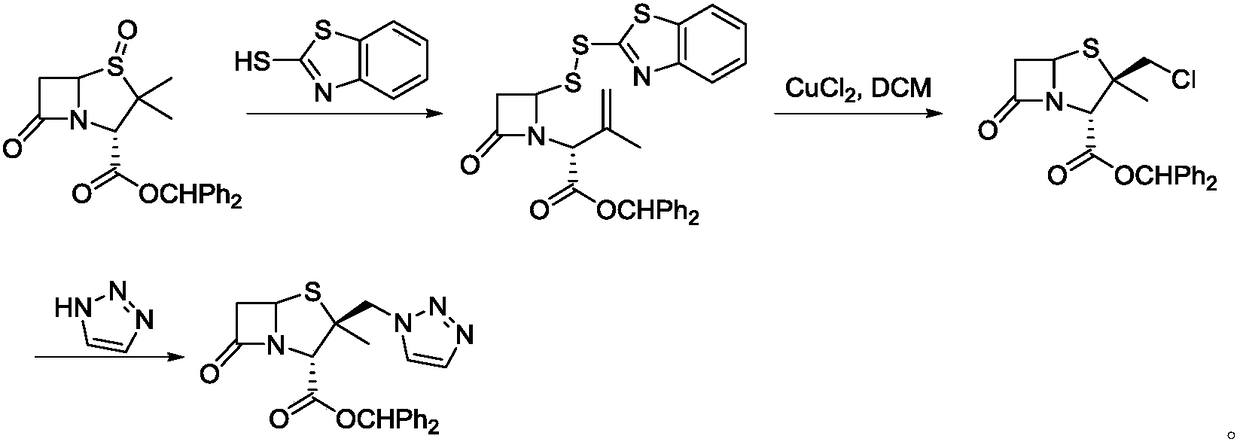
[0024] As described in the background technology, the existing methods for synthesizing tazobactam diphenylmethyl ester have the problems of many by-products, low product yield and serious environmental pollution. In order to solve the above-mentioned technical problems, the application provides a synthetic method of tazobactam diphenylmethyl ester, the synthetic method comprising: under the action of a catalyst, compound A and hydrogen peroxide are oxidized to obtain tazobactam diphenylmethyl ester. Benzyl ester, the synthetic route is as follows:
[0025]
[0026] In the above synthesis method, the tazobactam diphenylmethyl ester product is precipitated in solid form, and the desired product can be obtained only through filtration after the reaction is completed. In the synthesis method provided by this application, hydrogen peroxide is used as an oxidant, and the only by-product is water. This can greatly reduce the generation of waste solids, greatly reduce the amount o...
Embodiment 1
[0039]
[0040] At 20-25°C, compound A (10 g, 23.0 mmol) was placed in a 250 mL four-necked flask, and then acetone (50 mL) was added, and the system became light yellow and clear. The above system was heated up to 50°C, and the temperature was controlled at about 50°C, and then 35% hydrogen peroxide (6.70g, 69.0mmol) and Na 2 WO 4 .2H 2 O (0.152g, 0.46mmol), continue to insulate and stir after dropping, the system is milky white and turbid, and HPLC tracks the reaction process until the reaction of the raw materials is completed.
[0041] After the reaction is completed, the temperature of the product system is lowered to 20-25° C., and 10 wt % sodium sulfite aqueous solution is added dropwise to the above system to quench the remaining hydrogen peroxide. Then the system was cooled to 0-5° C. and then filtered to obtain 9.67 g of a white solid product after drying, with a yield of 95.7 wt % and a purity of 98.1 %.
[0042] The spectral data of the white solid product is...
Embodiment 2
[0044] The difference from Example 1 is that the molar ratio of compound A to the hydrogen peroxide is 1:2.
[0045] The yield of tazobactam diphenylmethyl ester was 85.4wt%, and the purity was 90.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com