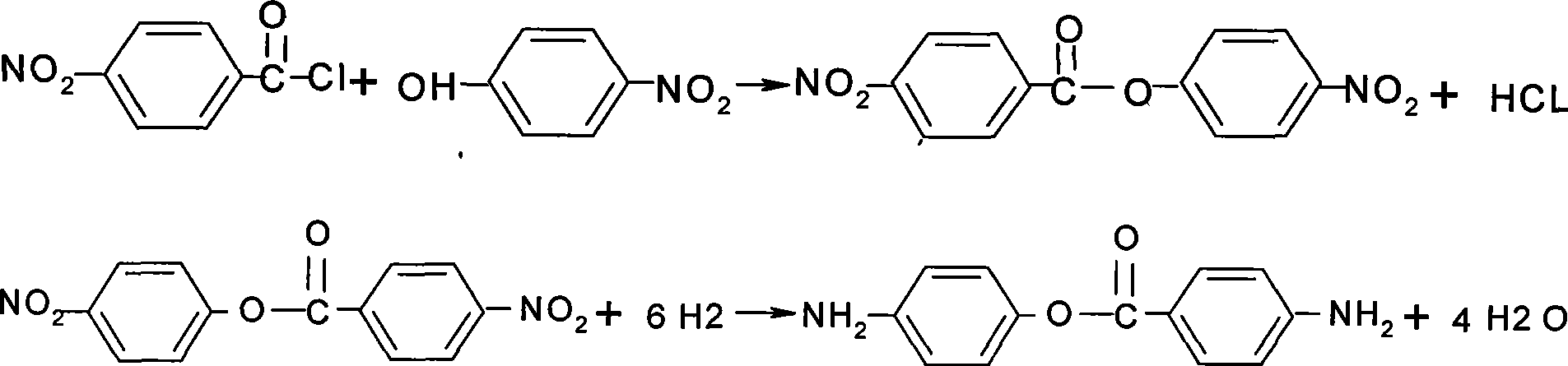
Preparation of 4-aminobenzoic acid (4-aminophenyl)-ester
A technology of aminobenzoic acid and aminophenyl, which is applied in the field of preparation of 4-aminobenzoic acid ester, can solve difficult problems and achieve the effects of low production cost, strong market competitiveness, and low environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In a 500ml three-neck flask, add 56g of p-nitrophenol, 43g of p-nitrobenzoyl chloride, 350ml of dimethylformamide DMF, 3g of KI, start stirring and heat up to 140°C, and carry out heat preservation and reflux reaction. The HCl gas generated is water Absorb into hydrochloric acid solution, react for 8 hours until the reaction is completely stopped, cool down and cool until the product precipitates crystals and carry out suction filtration. The filter cake is a dinitro intermediate of light yellow sand-like particles, and the component of the dinitro intermediate is 4-nitro 4-nitrobenzoic acid (4-nitrophenyl) ester, for the hydrogenation reaction that can be directly used in the next step, the purity of 4-nitrobenzoic acid (4-nitrophenyl) ester is 98.5%, and the whole condensation reaction The yield of the product is 97%, and the melting range of the dinitro intermediate is 160-163°C, which prevents HCl from siphoning in the process of water absorption.
[0022] Add 40g o...
Embodiment 2
[0024] In a 500 ml three-necked flask, add 56 g of p-nitrophenol, 48 g of p-nitrobenzoyl chloride, 250 ml of xylene, and 3 g of dimethylaminopyridine DMAP catalyst, start stirring and heat up to 135 ° C, and carry out heat preservation and reflux reaction, and the generated HCl The gas is absorbed into hydrochloric acid solution with water, and the reaction is completely stopped after 3 hours of reaction. The temperature is lowered and cooled until the product precipitates crystals, and suction filtration is performed. The filter cake is the dinitro intermediate of light yellow sand-like particles, 4-nitrobenzoic acid (4 -Nitrophenyl) ester has a purity of 98%, and the yield of the entire condensation reaction is 93%, preventing siphoning during HCl water absorption.
[0025] Add 40g of dinitro intermediate, 150ml of ethanol, and 5g of Pd / C catalyst with a palladium content of 3% into the autoclave, stir and heat up to 80°C, and the pressure rises to 2.0MPa for hydrogenation re...
Embodiment 3
[0027] In a 500ml three-neck flask, add 56g of p-nitrophenol, 38g of p-nitrobenzoyl chloride, 150ml of toluene, and 2g of catalyst KCl, start stirring and heat up to 115°C, and carry out heat preservation and reflux reaction, and the generated HCl gas is absorbed by water to form hydrochloric acid solution, stop the reaction after 3 hours of complete reaction, lower the temperature and cool until the product precipitates crystals and carry out suction filtration, the filter cake is the dinitro intermediate of light yellow sand-like particles, the yield of the entire condensation reaction is 93%, and the dinitro intermediate The melting range of the solid is 160-163°C.
[0028] Add 40g of dinitro intermediate, 250ml of methanol, and 0.5g of Raney-Ni catalyst into the autoclave, stir and raise the temperature and pressure to 80°C and 0.8MPa for catalytic hydrogenation reaction, filter the Raney-Ni catalyst, and distill the filtrate to obtain Yellow loose product 4-aminobenzoic a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting range | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com