Method for producing alcohol compound having fluorene skeleton
A technology of alcohol compounds and manufacturing methods, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve the problems of removing mercaptans, insufficient improvement of coloring, etc., and achieve the effect of less coloring
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0104] Hereinafter, although an Example etc. are given and this invention is demonstrated concretely, this invention is not limited at all. In addition, in the example, various measurements were implemented by the following method. In addition, the production rate (survival rate) and purity of each component described in the following Examples, Comparative Examples, and Reference Examples are HPLC area percentage values measured under the following conditions (excluding the solvent in the reaction solution and the included The corrected area percentage value after the peak of the organic compound), and the yield is the apparent yield when the obtained alcohol represented by the above formula (1) is an inclusion body and assumed not to be an inclusion body. In addition, the so-called "polymer" in Examples and Comparative Examples means compounds obtained by further reacting one molecule or more of ethylene carbonate on the alcohol compound represented by the above formula (1)...
Embodiment 2
[0148] Add 150g (0.298mol) of 9,9'-bis(4-hydroxy-3-phenylphenyl)fluorene, 3.4g (0.025mol) of potassium carbonate to a glass reactor equipped with a stirrer, a heating cooler, and a thermometer ), 60.1 g (0.682 mol) of ethylene carbonate, 225 g of toluene, and 150 g of diethylene glycol dimethyl ether, the temperature was raised to 115° C., and after stirring at the same temperature for 13 hours, it was confirmed by HPLC that the peak of the raw material disappeared. The formation rate of the polymer at the end of the reaction was about 0.5%.
[0149] After cooling the obtained reaction liquid to 85 degreeC, 225 g of water was added, it stirred at 80-85 degreeC for 30 minutes, and after standing still, the water layer was separated. After repeating the same operation three times, the obtained organic solvent layer was partially concentrated to obtain a solution containing the alcohol compound represented by the above formula (1), toluene, and diglyme.
[0150] To this solution...
Embodiment 3~6
[0161] The reaction and post-processing were performed similarly to Example 1, and the concentrate was obtained. The obtained concentrate was divided into 4 equal parts, and toluene and methyl alcohol were added respectively so as to reach the ratio shown in the following Table 1, and crystallization and drying operations were carried out in the same manner as in the method described in Example 1 to obtain the formula (1) represented by the above formula (1). crystals of alcohol compounds. Each analysis value of each crystal is shown in Table 1 below. In addition, the addition amount of toluene and methanol in Table 1 is the ratio (weight ratio) with respect to the alcohol compound represented by said formula (1) contained in each concentrate.
[0162] [Table 1]
[0163] [Table 1]
[0164]
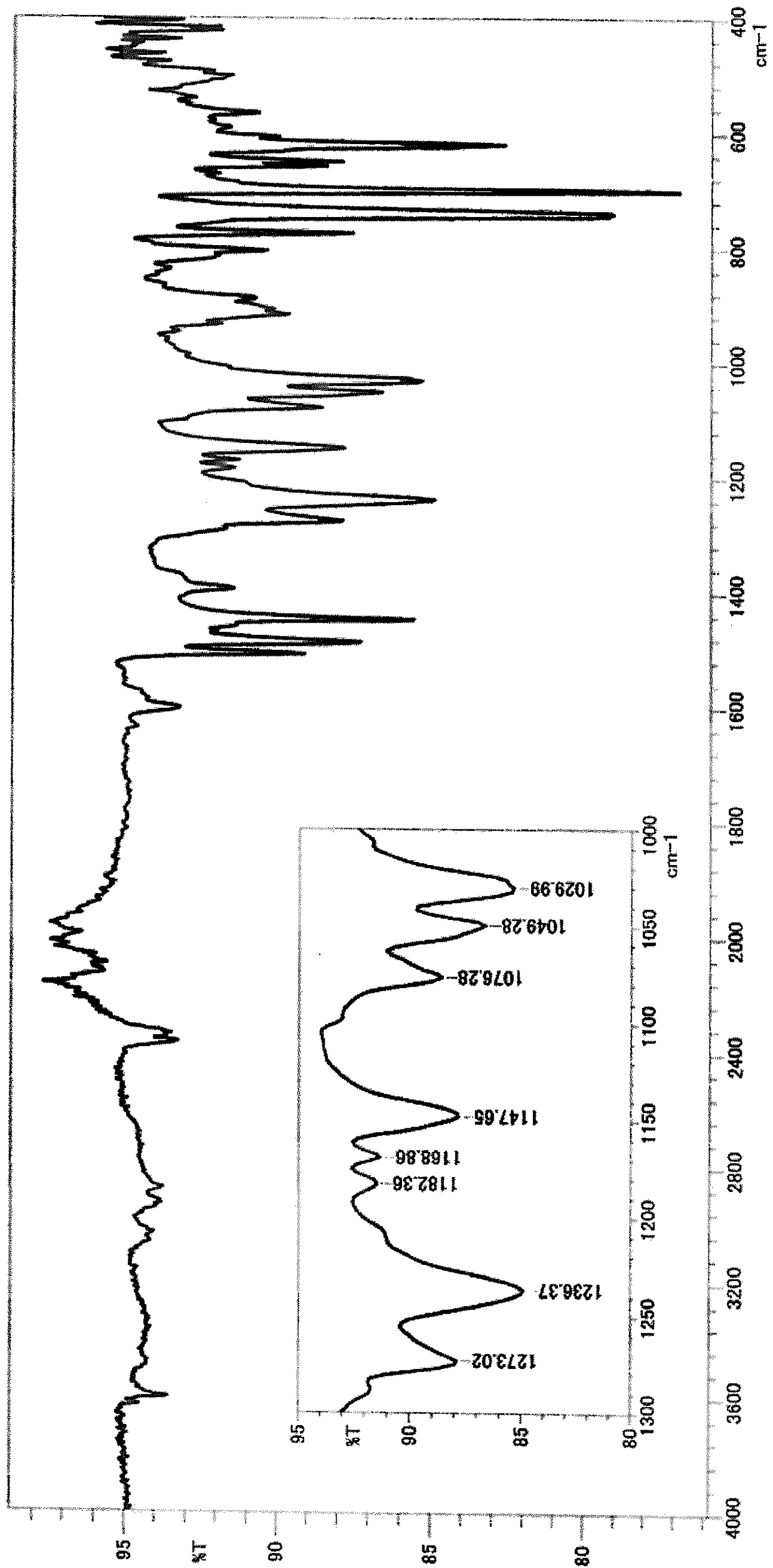
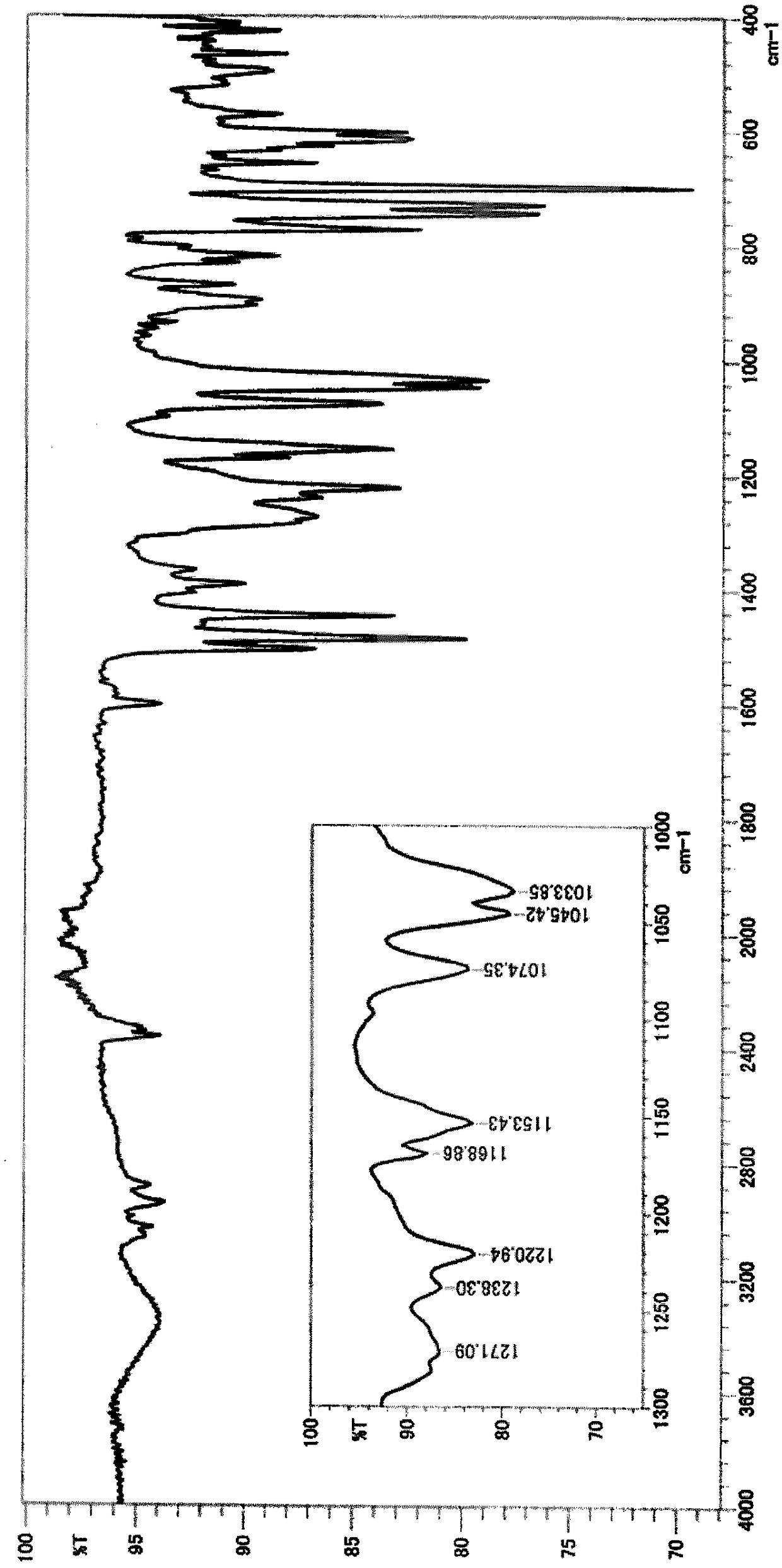
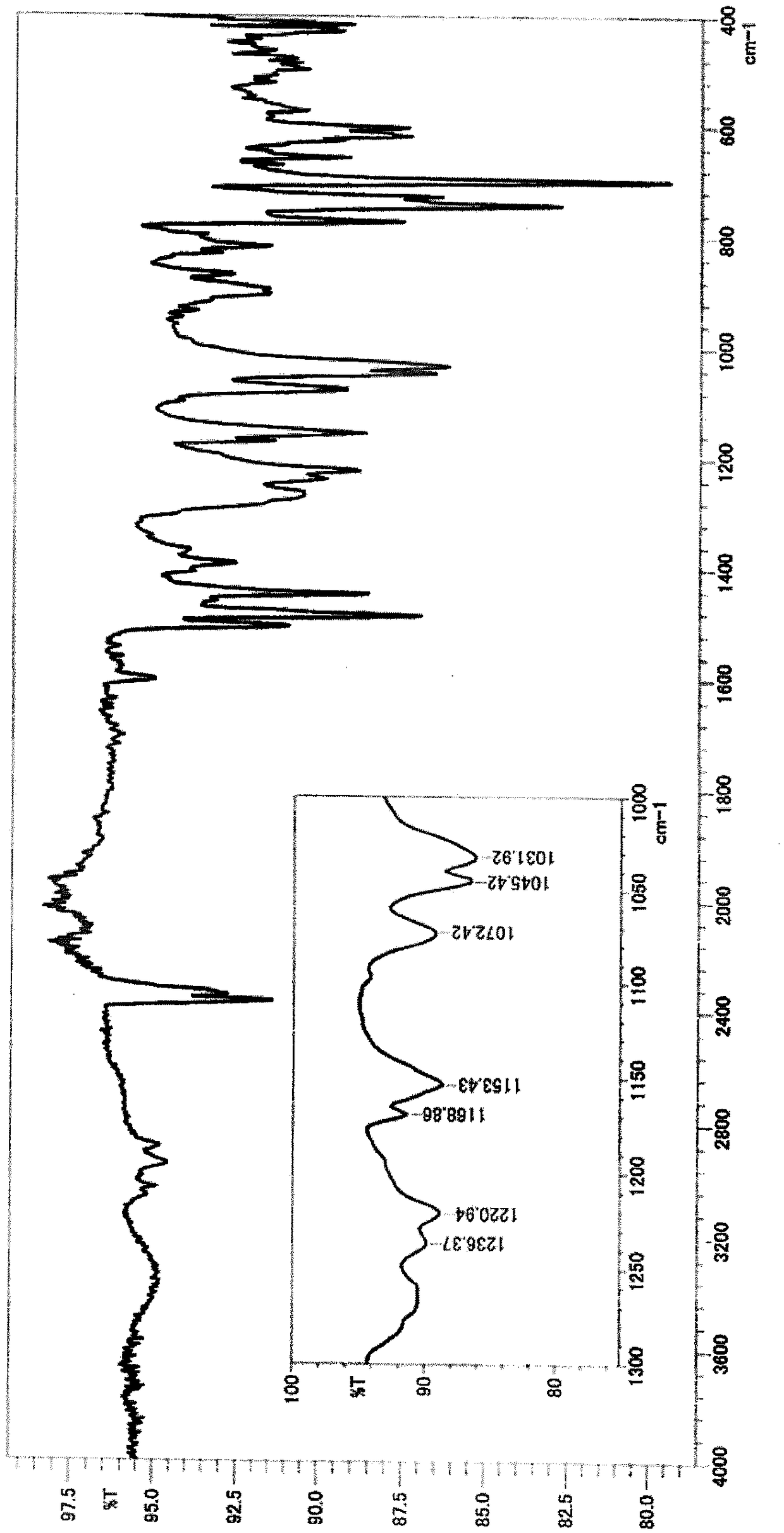
[0165] *1 above area (cm -1 ) in the presence or absence of peaks.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com