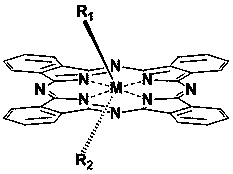
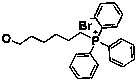
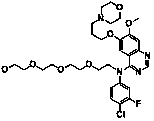
Double-targeted phthalocyanine anti-cancer photosensitizer and preparation method thereof
A technology for phthalocyanines and photosensitizers, which is applied in the field of asymmetrically substituted phthalocyanine complexes with aza aromatic rings and the preparation thereof, and achieves the effects of low cost, mild reaction conditions and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation method of the asymmetrically substituted phthalocyanine complexes with tumor-specific and mitochondrial localization targets, the experimental steps are as follows:
[0035] (1) Synthesis of 1a
[0036] First, the compounds tetraethylene glycol (9.706 g, 50.0 mmol) and p-toluenesulfonyl chloride (2.387 g, 12.5 mmol) were added to a 250 ml round bottom flask, followed by anhydrous CH 2 Cl 2(150 ml), fully stirred and dissolved, then added triethylamine (6.324g, 62.5 mmol), and reacted at room temperature under nitrogen protection for 12 h; after the reaction, extracted with 1mol / L hydrochloric acid solution and saturated sodium chloride solution respectively Three times, the organic phase was collected, dried over anhydrous sodium sulfate, and the CH was removed under reduced pressure 2 Cl 2 , and then eluted with dichloromethane and methanol, and separated by silica gel column chromatography to obtain compound 1a 2-(2-(2-(2-hydroxyethoxy)ethoxy)ethoxy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com