Iron and nitrogen co-doped carbon-oxygen reduction catalyst, and preparation method and application thereof
A co-doping and catalyst technology, applied in structural parts, electrical components, battery electrodes, etc., can solve the problems of unfavorable reactant and product mass transfer, metal nanoparticles precipitation and agglomeration, reduction of active site utilization, etc. The reduction catalytic activity and electrochemical stability are excellent, the preparation method is simple, and the effect of the optimization of the preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
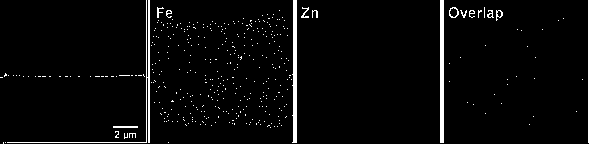
[0037] Using Fe, Zn-ZIF as a precursor for high-temperature pyrolytic carbonization to prepare an iron-nitrogen co-doped carbon oxygen reduction catalyst includes the following steps:
[0038] (1) Zn(NO 3 ) 2 ·6H 2 O (788.31 mg, 2.38 mmol) and FeSO 4 ·7H 2 O (33.1 mg, 0.12 mmol) was dissolved in 90 mL of methanol, while benzimidazole (1180 mg, 10 mmol) was dissolved in 90 mL of methanol, ultrasonically dissolved and dispersed evenly, and the benzimidazole containing The methanol solution is poured into the methanol solution of the metal salt, and the solution mixing rate is 9mL s -1 ; Stir at room temperature for 1 min after mixing, pour into a 200 mL reaction kettle, and then transfer to an oven at 130 o C for 24 h, cooling, suction filtration and repeated washing with organic solvents, and then drying to obtain a yellow powder. Among them, Zn(NO 3 ) 2 ·6H 2 O and FeSO 4 ·7H 2 O molar ratio is 20, metal salt and benzimidazole molar ratio is 0.25, and the concentrat...
Embodiment 2
[0042] The preparation process was similar to that of Example 1, except that the hydrothermal reaction time was reduced to 16 h during the preparation of the precursor Fe,Zn-ZIF. SEM image ( Figure 7 ) shows that the prepared Fe,Zn-ZIF has a cylindrical shape with an average diameter of about 6.5 μm, and the oxygen reduction performance test of the catalyst prepared by pyrolytic carbonization ( Figure 8 ) shows that the catalyst has better electrocatalytic activity than commercial 20%Pt / C, and the half-wave potential exceeds 40 mV, which is about 10 mV lower than that in Example 1.
Embodiment 3
[0044] A preparation process similar to that of Example 1 was adopted, except that, in the preparation process of the precursor Fe, Zn-ZIF, the molar ratio of metal salt to benzimidazole was 0.125. SEM image ( Figure 9 ) shows that the morphology of the prepared Fe, Zn-ZIF is cylindrical, and the aspect ratio is reduced from 1:1 to 1:2. The oxygen reduction performance test of the catalyst prepared by pyrolysis carbonization ( Figure 10 ) shows that the catalyst has better electrocatalytic activity than commercial 20% Pt / C, and the half-wave potential exceeds 35 mV, which is about 15 mV lower than that in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com