Preparation method of octreotide acetate microspheres
A technology of octreotide acetate and microspheres, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, and pharmaceutical formulas, can solve problems such as hindering the use of drugs, uneven distribution of microspheres, aggregation, etc. Fast distribution, simple operation, and simple method steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
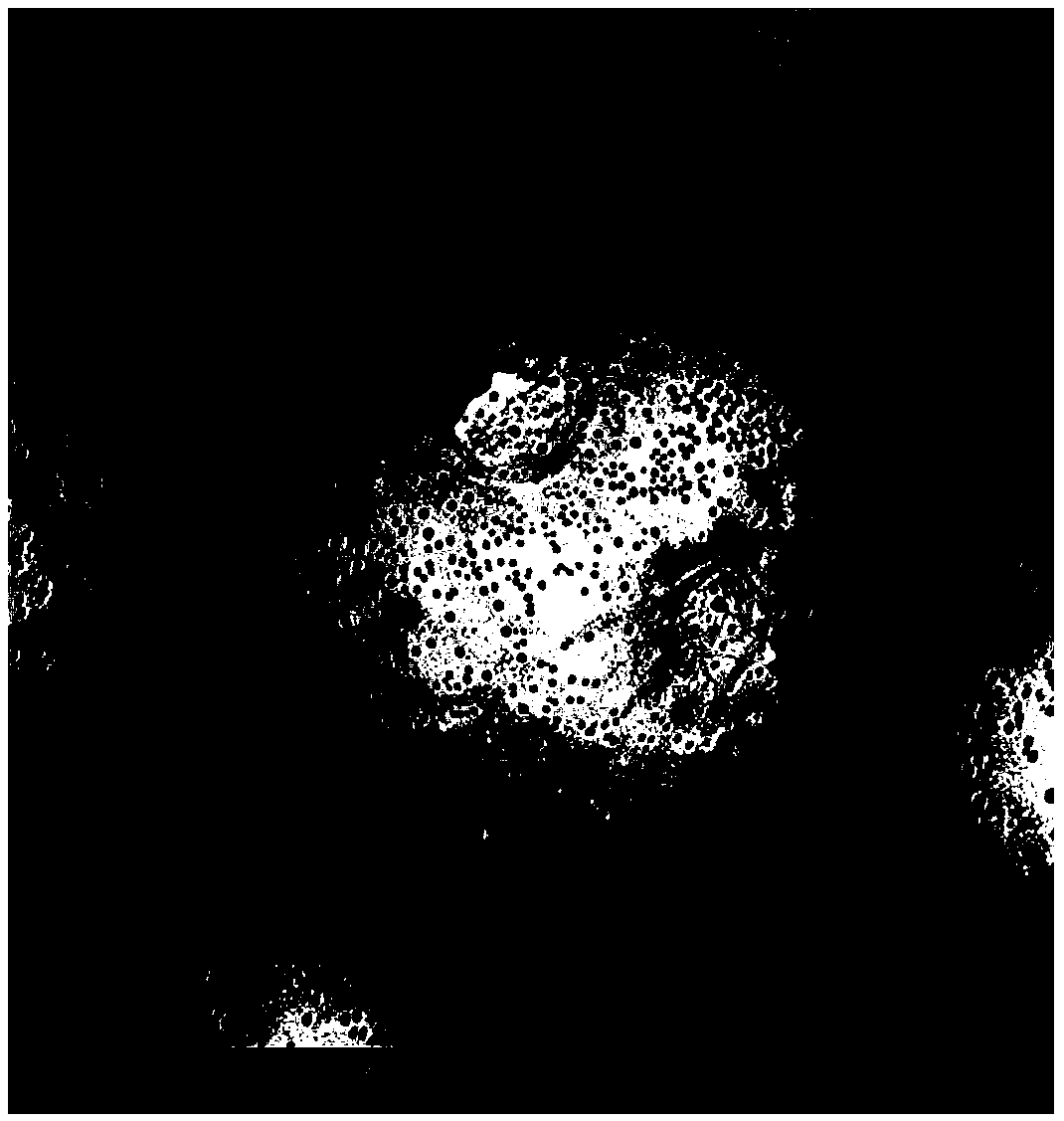
[0049] The octreotide acetate that takes 0.75g is dissolved in 15g methanol; The PLGA that gets 10g is dissolved in 400g methylene chloride; The above two parts of solutions are stirred and mixed, rated speed 120rpm, form homogeneous solution; Get 640ml silicone oil (viscosity 350cs), in After adding at a constant speed within 13 minutes, continue to stir for 5 minutes, and control the temperature at 8-10°C to form agglomerates; Solidify, solidify for 2 hours; form microspheres after solidification, collect by filtration, wash with n-heptane for 3-4 times, add appropriate amount of mannitol solution, dry in vacuum, and sieve to obtain the product. See SEM image figure 1 and 2 .
[0050] The accelerated release results of the resulting product are as follows:
[0051] Table 1-1
[0052]
[0053]
[0054] Note: The result is the accumulative accelerated release degree of accelerated release 1h, 4h and 24h
[0055] The particle size distribution of the product obtained...
Embodiment 2
[0061] The octreotide acetate that takes 0.75g is dissolved in 15g methanol; The PLGA that gets 10g is dissolved in 345g methylene chloride; The above two parts of solutions are stirred and mixed, rated speed 120rpm, form homogeneous solution; Get 640ml silicone oil (viscosity 350cs), in After adding at a constant speed within 13 minutes, continue to stir for 5 minutes, and control the temperature at 8-10°C to form agglomerates; add a solidifying solution composed of n-heptane (3200ml), water (400ml), Span80 (20ml), and silicone oil (100ml) to solidify , solidified for 2 hours; microspheres formed after solidification, collected by filtration, washed with n-heptane for 3-4 times, added an appropriate amount of mannitol solution, dried in vacuum for 20 hours, and sieved to obtain the product. See SEM image image 3 and 4 .
[0062] The accelerated release results of the resulting product are as follows:
[0063] table 2-1
[0064]
Drug loading
1h
4h
2...
Embodiment 3
[0069] The octreotide acetate that takes 0.75g is dissolved in 15g methanol; The PLGA that gets 10g is dissolved in 400g methylene chloride; The above two parts of solutions are stirred and mixed, rated speed 120rpm, form homogeneous solution; Get 640ml silicone oil (viscosity 600cs), in After adding at a constant speed within 13 minutes, continue to stir for 5 minutes, and control the temperature at 8-10°C to form agglomerates; Solidify, solidify for 2 hours; form microspheres after solidification, collect by filtration, wash with n-heptane for 3-4 times, add appropriate amount of mannitol solution, dry in vacuum for 20 hours, and sieve to obtain the product. See SEM image Figure 5 and 6 .
[0070] The accelerated release results of the resulting product are as follows:
[0071] Table 3-1
[0072]
Drug loading
1
4
24
W03-171113-2-0-1
4.72%
2.51%
28.55%
54.97%
[0073] The particle size distribution of the product obtained is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com