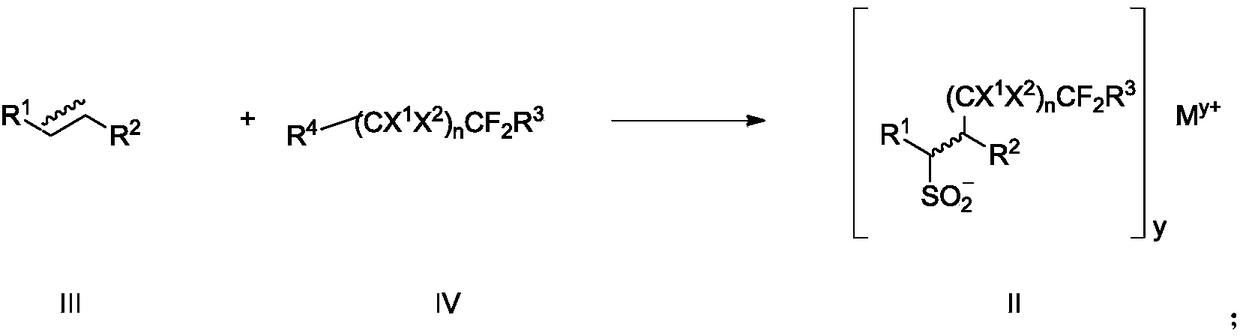
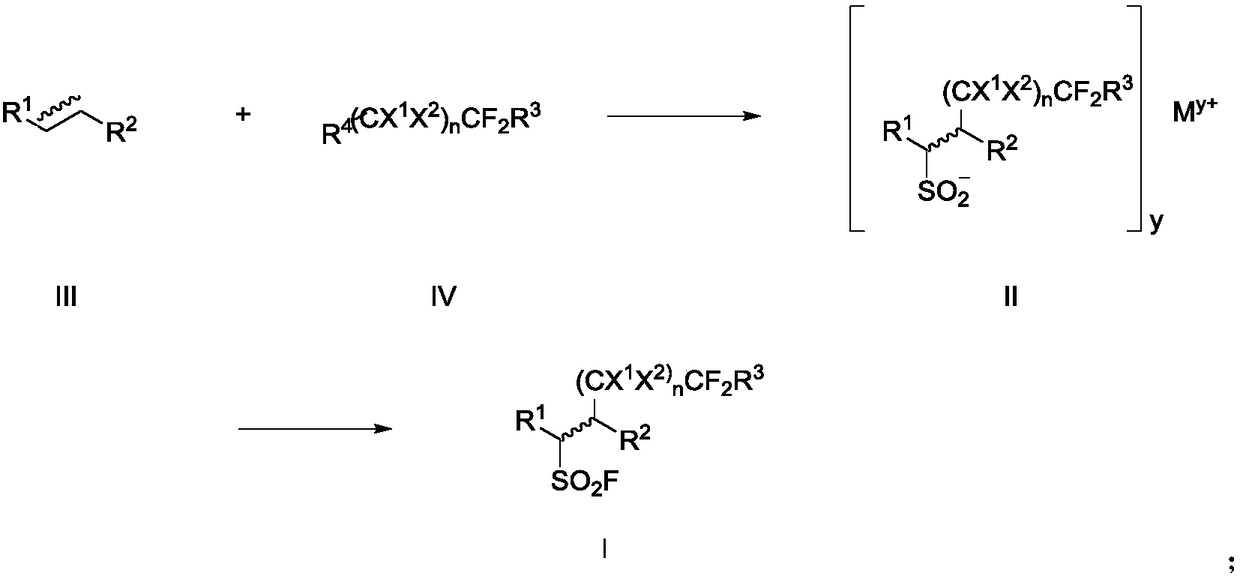
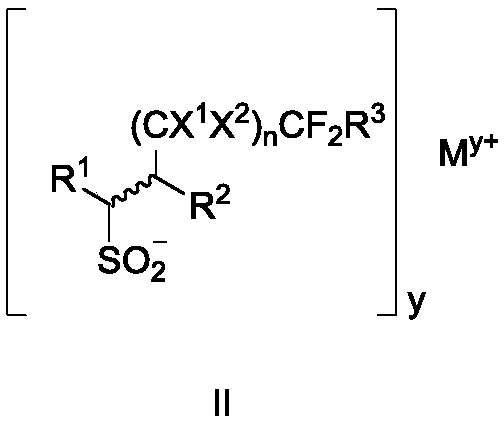
Fluoroalkyl sulfonyl fluoride compound as well as intermediate, preparation method and application thereof
A compound and fluoroalkyl technology, which is applied in the preparation of sulfonic acid, organic chemistry, drug combination, etc., can solve the problems of small fluoroalkyl substrates, high toxicity, and volatility of reagents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120]
[0121] Zinc powder (0.3mmol) and sodium dithionite (0.6mmol) were added to the reaction flask, the argon was replaced three times, and DMF (4.5mL), H 2 O (75 uL), 4-phenyl-1-butene (0.3 mmol) and ethyl difluorobromoacetate (0.6 mmol). The reaction flask was placed at 25°C and stirred for 10 hours. NFSI (1.2 mmol) was added under the protection of argon, and stirred at room temperature for 3 h. With 4-(trifluoromethoxy)anisole as internal standard substance, using 19 F-NMR confirmed the formation of the target product. The reaction solution was filtered, and the filtrate was used to remove the solvent by a rotary evaporator, separated by column chromatography, and concentrated under reduced pressure to obtain a light yellow liquid (51% yield). The relevant data are as follows: 1 H NMR (400MHz, CDCl 3 ):δ7.33(t, J=7.3Hz, 2H), 7.28–7.24(m, 1H), 7.23–7.19(m, 2H), 4.36(q, J=7.1Hz, 2H), 3.81–3.73( m,1H),3.01–2.83(m,3H),2.65–2.51(m,1H),2.49–2.37(m,1H),2.34–2.25(m,1H...
Embodiment 2
[0123]
[0124] Zinc powder (0.6mmol) and DABSO (0.6mmol) were added to the reaction flask, the argon gas was replaced three times, and DMF (4.5mL), H 2 O (75 uL), 4-phenyl-1-butene (0.3 mmol) and ethyl difluorobromoacetate (0.6 mmol). The reaction flask was placed at -5°C and stirred for 6 hours. NFSI (1.2 mmol) was added under the protection of argon, and stirred at room temperature for 3 h. With 4-(trifluoromethoxy)anisole as internal standard substance, using 19 F-NMR confirmed the formation of the target product. Filter the reaction solution, wash the solid impurities with dichloromethane, wash three times with water, wash once with saturated brine, dry the organic phase with anhydrous sodium sulfate, remove the solvent by rotary evaporator after filtration, separate by column chromatography, and concentrate under reduced pressure to obtain light yellow Liquid (69% yield). Relevant data see embodiment 1.
Embodiment 3
[0126]
[0127] Zinc powder (0.6mmol) and DABSO (0.6mmol) were added to the reaction flask, the argon gas was replaced three times, and DMF (4.5mL), H 2 O (75 uL), 4-phenyl-1-butene (0.3 mmol) and N,N-diethyldifluorobromoacetamide (0.6 mmol). The reaction flask was placed at -5°C and stirred for 12 hours. NFSI (1.2 mmol) was added under the protection of argon, and stirred at room temperature for 3 h. With 4-(trifluoromethoxy)anisole as internal standard substance, using 19 F-NMR confirmed the formation of the target product. Filter the reaction solution, wash the solid impurities with dichloromethane, wash three times with water, wash once with saturated brine, dry the organic phase with anhydrous sodium sulfate, remove the solvent by rotary evaporator after filtration, separate by column chromatography, and concentrate under reduced pressure to obtain light yellow Liquid (66% yield). The relevant data are as follows: 1 H NMR (400MHz, CDCl 3):δ7.31(t,J=7.3Hz,2H),7.26...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com