Perylene diimide hexamer compound and preparation method, composition and organic solar cell
A perylene diimide and solar cell technology, applied in the field of solar cells, can solve the problems of poor absorption of visible light, difficulty in chemical modification of fullerene derivatives, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] HPB-PDI 6 Synthesis of receptor molecules
[0039] HPB-PDI 6 The synthetic route of is shown below. Compounds 1 and 2 were prepared by existing literature. The preparation of compound 1 can refer to Y.Zhao, X.Li, Z.Wang, W.Yang, K.Chen, J.Zhao and G.G.Gurzadyan, J.Phys.Chem.C., 2018, 7, 3756-3772; The preparation of compound 2 can refer to M. Takase, A. Nakajima and T. Takeuchi, Tetrahedron Lett., 2005, 46, 1739-1742.
[0040]
[0041] Synthesis of Compound 3
[0042] Add compound 1 (485mg, 0.58mmol), compound 2 (100mg, 0.23mmol), solvent tetrahydrofuran (40mL) and water (4mL) in a 100mL round bottom flask, add catalyst Pd (PPh 3 ) 4 (0.012mmol, 13.4mg), and then reacted at a temperature of 80° C. for 72 hours under a nitrogen system. After stopping the reaction, evaporate the solvent, extract twice with dichloromethane, combine the organic layers, add anhydrous magnesium sulfate to dry, and remove the solvent to obtain the crude product, which is purified by ...
Embodiment 2
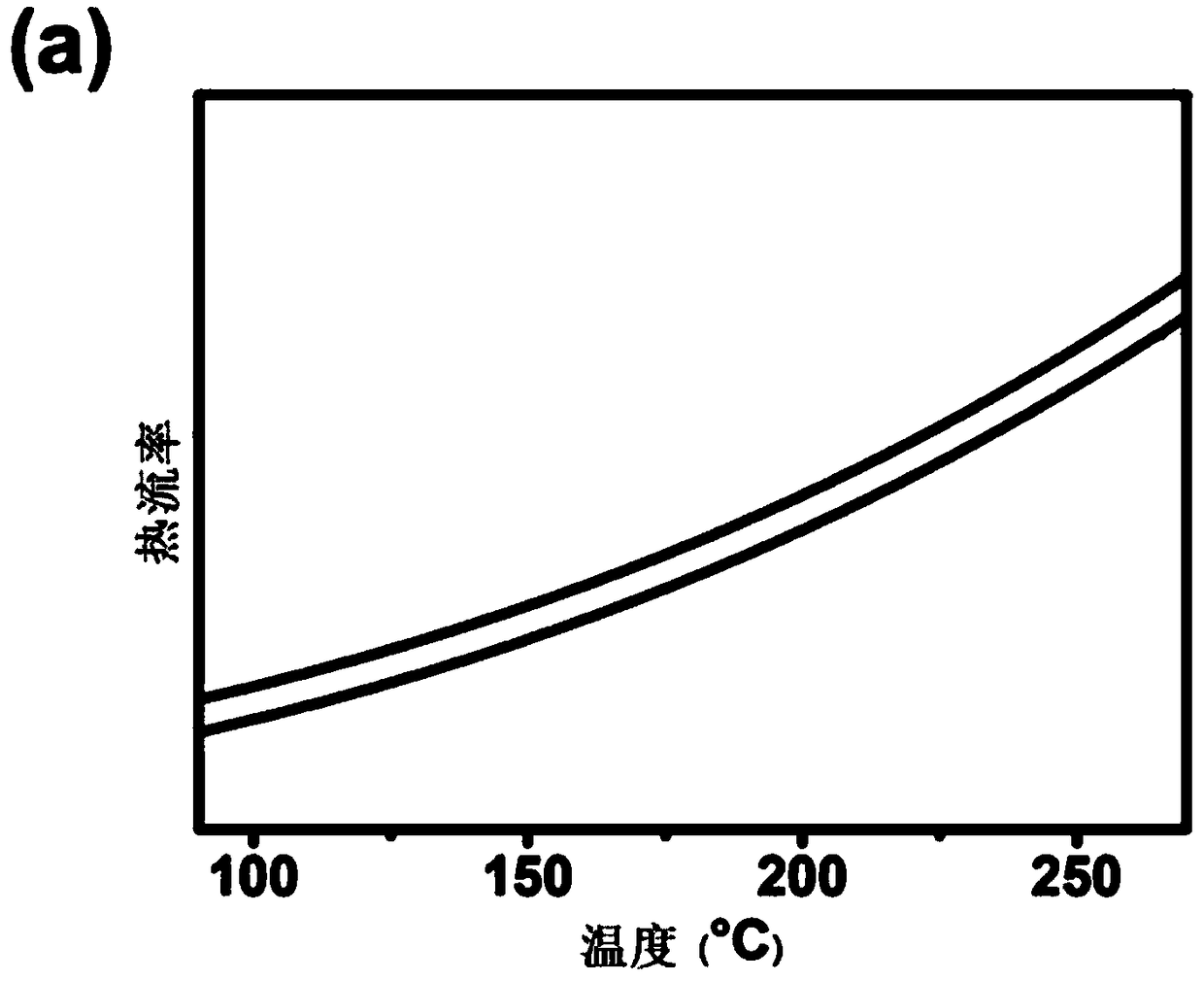
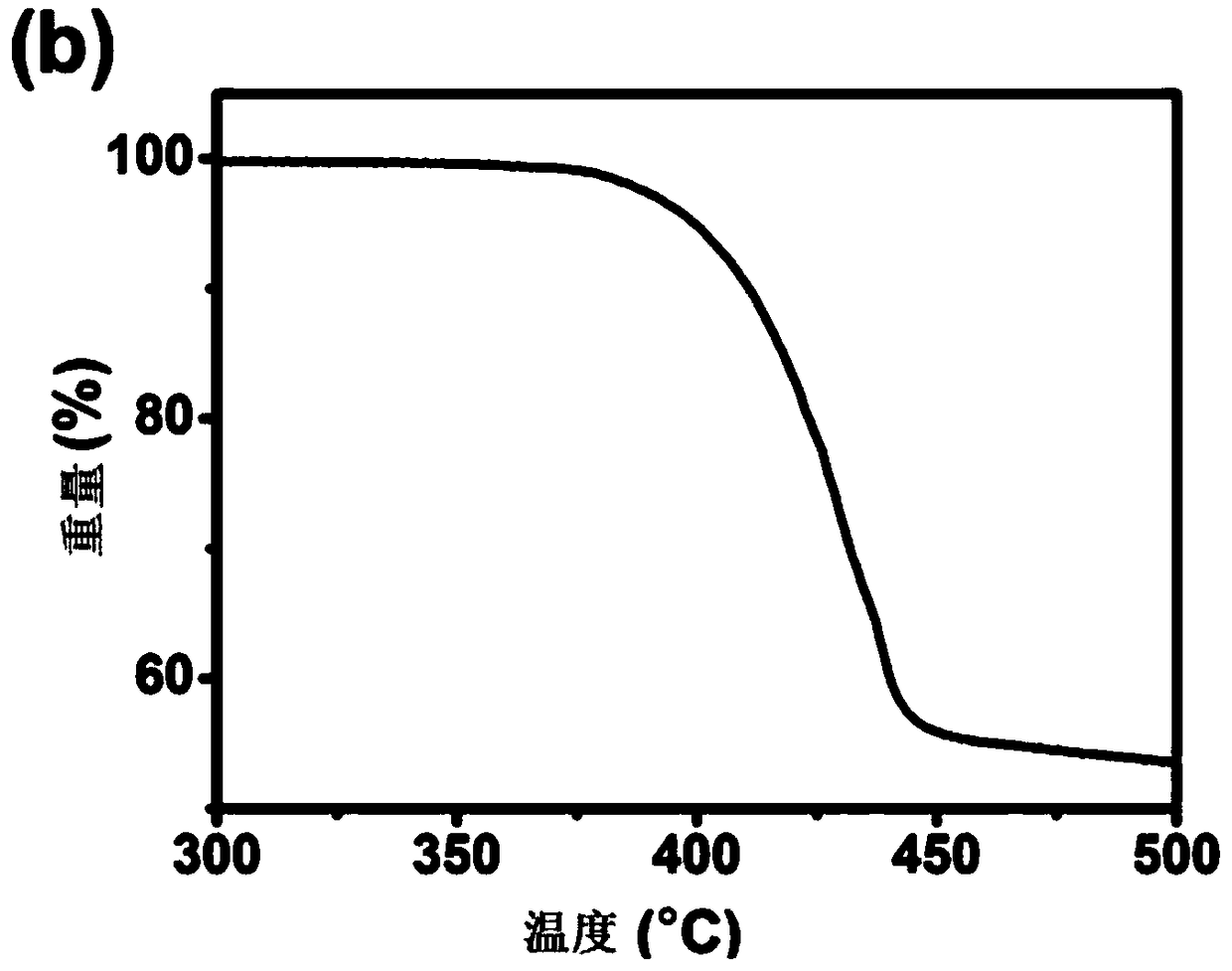
[0047] HPB-PDI 6 Thermodynamic Performance Analysis
[0048] Determination of HPB-PDI by Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC) 6 The thermodynamic properties of the thermodynamic properties are all measured under a nitrogen atmosphere and heated at a rate of 10°C / min. As shown in Figure 1(a), TGA showed that HPB-PDI 6 The temperature at which 5% mass loss occurs is 400°C, indicating that it has good thermal stability. As shown in Figure 1(b), in the range of 50°C-250°C, HPB-PDI 6 There are no obvious crystallization peaks or glass transitions in the DSC results, indicating that HPB-PDI 6 It is an amorphous small molecule that easily forms an amorphous film.
Embodiment 3
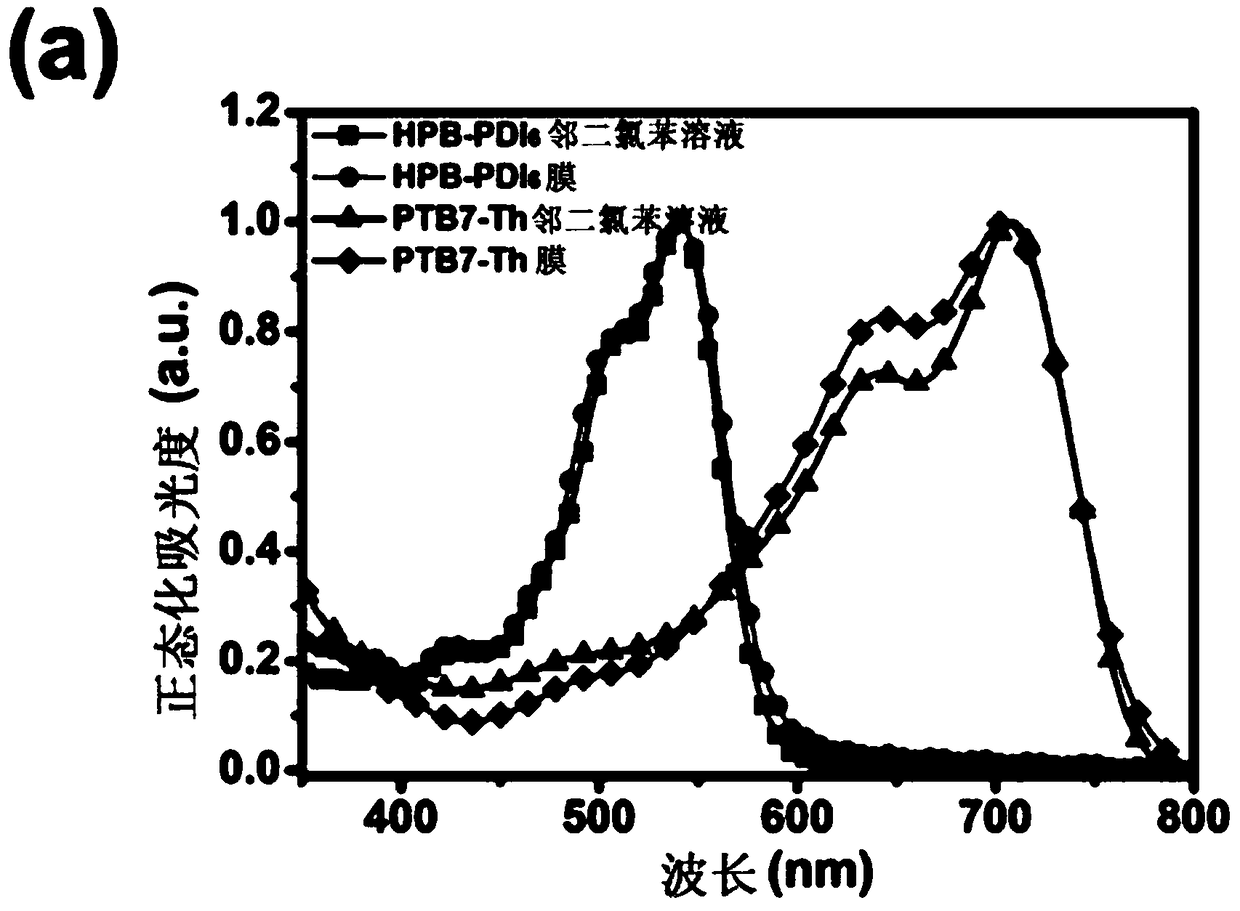
[0050] Electrochemical properties
[0051] HPB-PDI 6 The electrochemical properties were measured by cyclic voltammetry, and the cyclic voltammetry curves are shown in Figure 2(c) and Figure 2(d). Using ferrocene / ferrocene salt (Fc / Fc + ) redox pair as an internal standard, and the redox potential of ferrocene / ferrocene salt is 0.09eV. The calculation formula of HOMO and LUMO energy level is E HOMO =-e[E ox +4.80-E (Fc / Fc + ) ],E LUMO =-e[E re +4.80-E (Fc / Fc + ) ], HPB-PDI measured by cyclic voltammetry 6 The HOMO and LUMO energy levels of are -5.69 and -3.93eV, respectively. HPB-PDI 6 The energy level differences of the HOMO and LUMO of PTB7-Th are 0.47eV and 0.29eV, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com