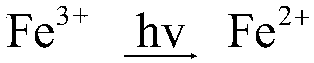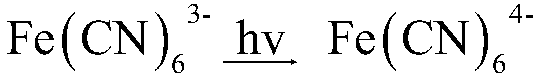Preparation method and application of illumination time indicating-capable gradient-ramp crack-free gel
A technology of light time and gradient color, which is applied in the field of preparation of gradient color-free gel without cracks, can solve problems such as cracking of gel materials, and achieve the effects of moderate hardness, uniform texture and wide application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Comparative Test
[0023] a) Preparation of traditional Gel
[0024] Put 2mL TEOS and 1mL 0.02mol / L HCl into a small beaker and seal it with parafilm. Stir with a magnetic stirrer for three hours at room temperature. Hole, store the sample and observe the phenomenon; through the experimental phenomenon, it is observed that the prepared gel is prone to cracking and bursting into many small fragments.
[0025] Through the analysis, it can be seen that due to the limitations of the organic reaction, the reaction is not complete, and the volatilization of some organic solvents, by-products, water and other substances that did not participate in the reaction causes the gel to crack when the gas escapes, but the main reason is that the prepared gel The glue structure skeleton is unstable and prone to collapse.
[0026] b) Preparation of blue gel (BL / Gel)
[0027] 2mL TEOS, 1mL 0.02mol / L HCl, 1mL 0.001mol / L K 3 Fe(CN) 6 , put it into a small beaker, and seal it with a par...
Embodiment 2
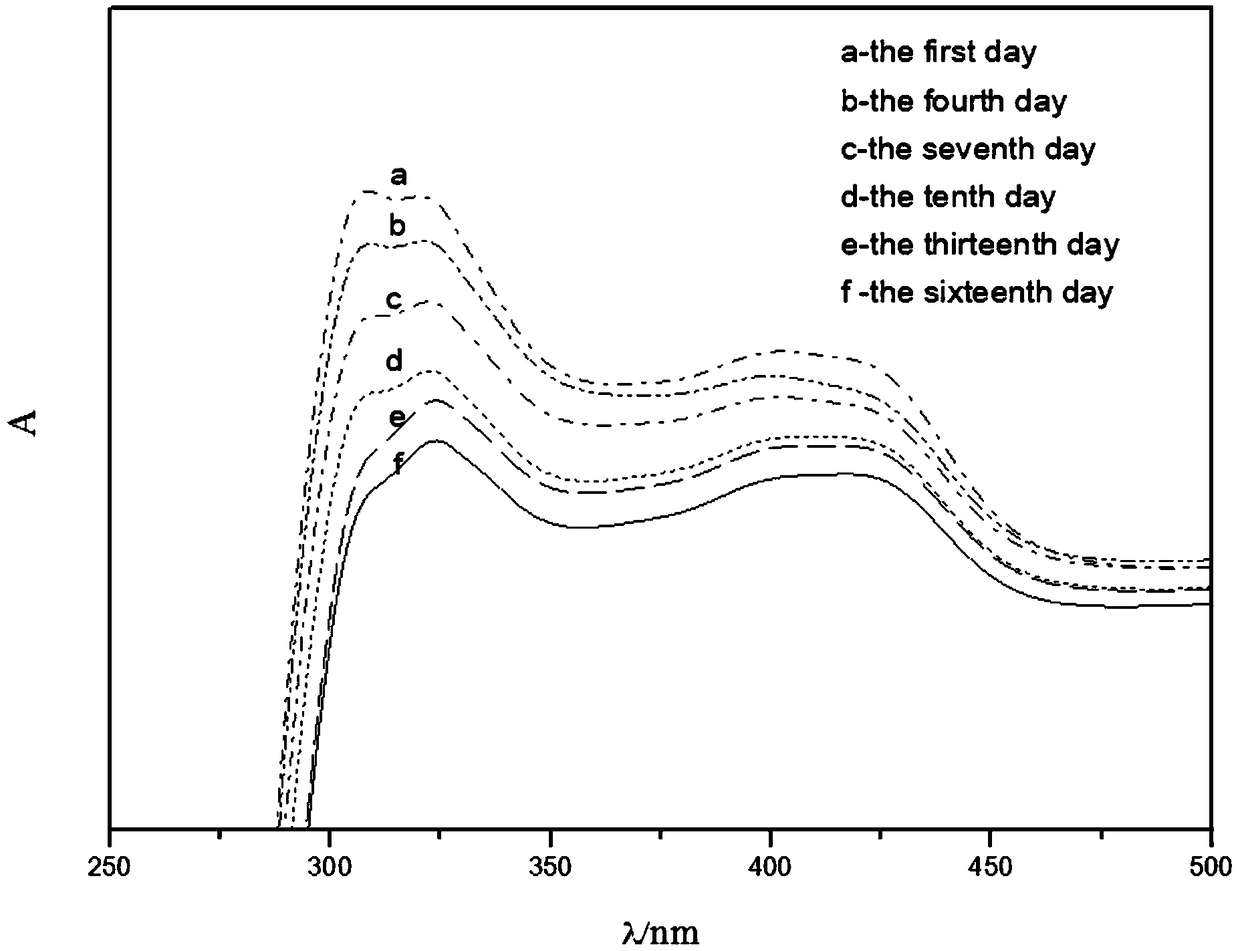
[0041] Effects of different experimental conditions on the preparation of BL-IL / Gel
[0042] a) Effect of pH value on the preparation of BL-IL / Gel
[0043] Prepare a 0.02mol / L HCl solution with a pH of 1.7, and then adjust the pH of the HCl solution to 2.3 and 3.4 with a pH indicator. In three small beakers add 2 mL TEOS, 1 mL 1.0 mM K 3 Fe(CN) 6 and 1mL 1-butyl-3-methylimidazolium tetrafluoroborate, then add 1mL HCl solution with pH of 1.7, 2.3, 3.4 respectively, and seal with parafilm, stir separately at room temperature with a magnetic stirrer Two hours, three hours, five hours, after the stirring is completed, use a thin needle to pierce some small holes on the parafilm, store the samples, and observe the phenomenon.
[0044] The experimental results found that when the hydrochloric acid solution with a pH value of 1.7 was added, a sol would be formed after stirring for two hours; when the pH value of the hydrochloric acid solution was added to 2.3, it would take three ...
Embodiment 3
[0059] Prepare light labels
[0060] Optimize K 3 Fe(CN) 6 Concentration, to prepare light labels for five-day color development
[0061] In this example, 0.5mM K 3 Fe(CN) 6 The color change of the gel under light conditions is an indicator signal, indicating a certain time span.
[0062] Contains 0.5mM K 3 Fe(CN) 6 When BL-IL / Gel is stored under light conditions and the storage time is different, the color that appears is also different. Prepare solution containing 0.5mM K 3 Fe(CN) 6 When BL-IL / Gel was left for one day, two days, or three days, the gel showed no obvious color change. It would appear blue when it was left for four days under light conditions. On the fifth day, the polycondensation discoloration was completely obtained and a uniform blue block without cracks was obtained. According to containing 0.5mM K 3 Fe(CN) 6 BL-IL / Gel develops color in four days and changes color in five days, which can be applied to food light labels to indicate the shelf life...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com