Electrochemical method for self-doping modification of titanium dioxide photo-catalyst
A technology of photocatalyst and titanium dioxide, which is applied in the direction of catalyst activation/preparation, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problems of Ti3+ unstable functional nanomaterials, and achieve green material preparation methods and speed up the reaction speed , to achieve the effect of catalyst regeneration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
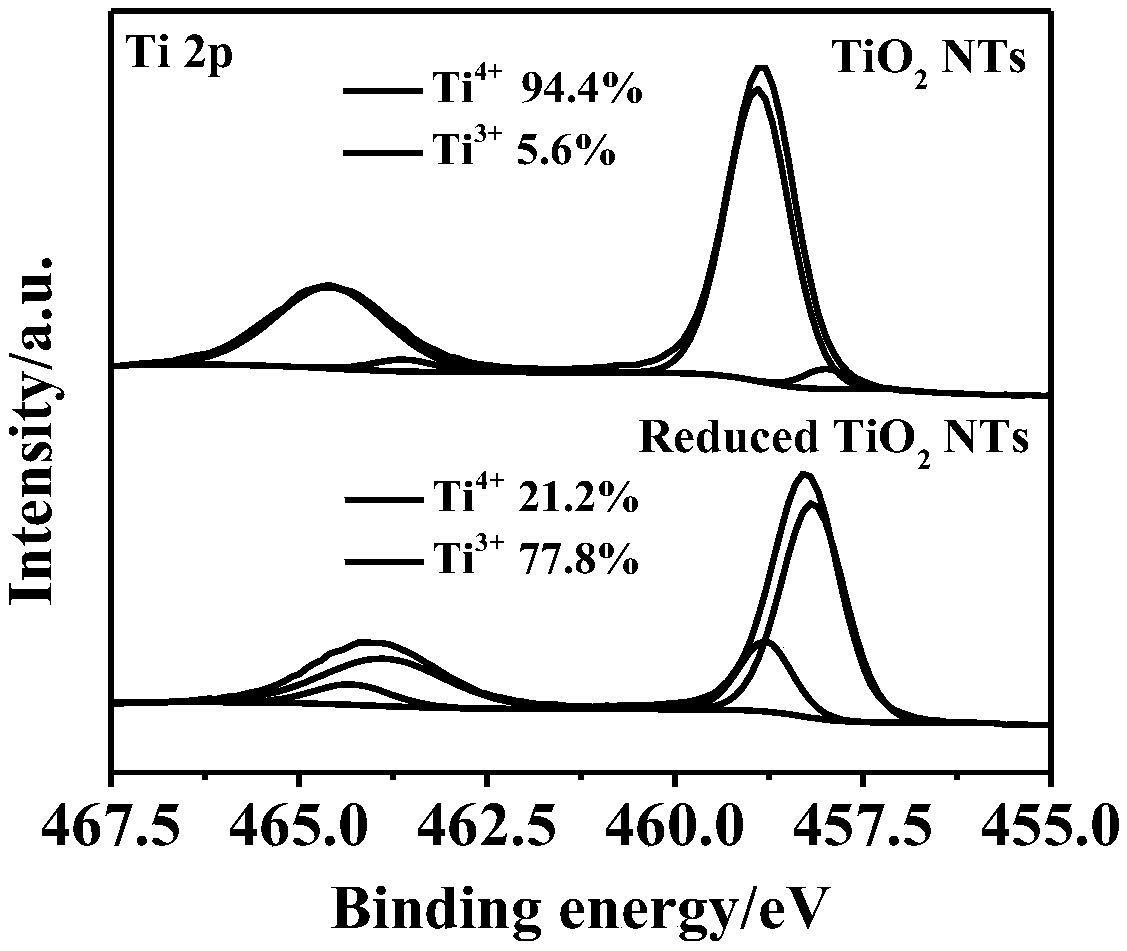
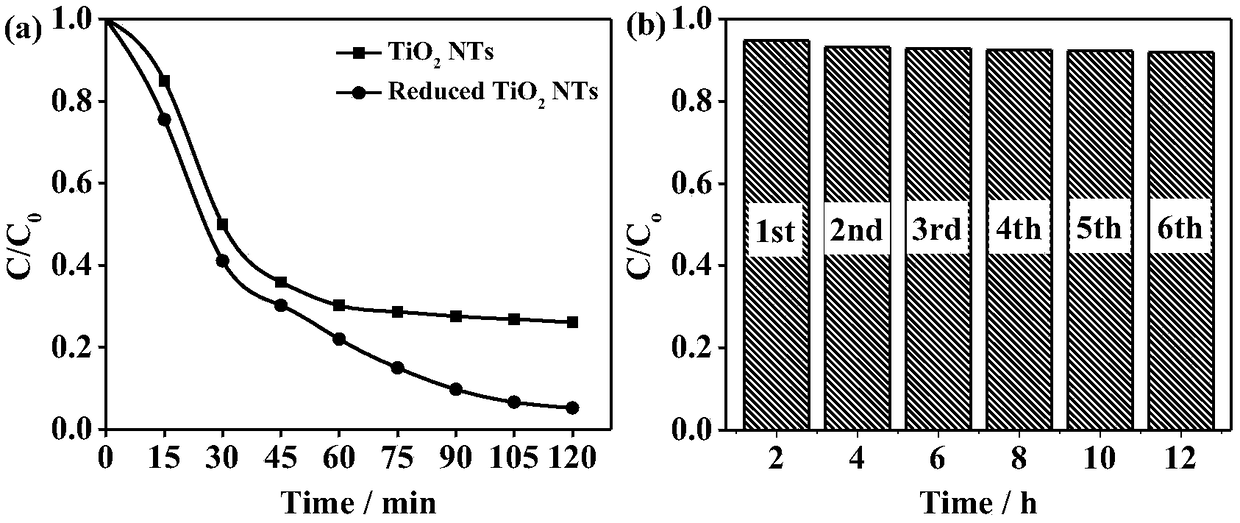
Image
Examples
Embodiment 1
[0025] (1) Pretreatment: Cut out a Ti sheet with a size of 1cm*4cm with scissors, ultrasonically clean it with ethanol, acetone, and deionized water in sequence to remove surface impurities, and dry it for later use;
[0026] (2) Anodic oxidation: the titanium sheet obtained in step (1) is used as the anode, the titanium sheet is used as the negative electrode, and the dimethyl sulfoxide solution containing hydrogen fluoride is used as the electrolyte to carry out anodic oxidation, and the mass fraction of hydrogen fluoride in the electrolyte is 2%. When preparing the electrolyte, first measure the dimethyl sulfoxide solution and pour it into a small plastic beaker, then measure the hydrogen fluoride solution and add it to the dimethyl sulfoxide solution, and finally magnetically stir the electrolyte for 3 minutes to obtain a well-mixed electrolyte system. The voltage of anodizing is 30V, and the time is 7h. After oxidation, the Ti sheet was rinsed with deionized water and dr...
Embodiment 2
[0030] (1) Pretreatment: Cut out a Ti sheet with a size of 1cm*4cm with scissors, ultrasonically clean it with ethanol, acetone, and deionized water in sequence to remove surface impurities, and dry it for later use;
[0031] (2) Anodic oxidation: the titanium sheet obtained in step (1) is used as the anode, the titanium sheet is used as the negative electrode, and the dimethyl sulfoxide solution containing hydrogen fluoride is used as the electrolyte to carry out anodic oxidation, and the mass fraction of hydrogen fluoride in the electrolyte is 2%. When preparing the electrolyte, first measure the dimethyl sulfoxide solution and pour it into a small plastic beaker, then measure the hydrogen fluoride solution and add it to the dimethyl sulfoxide solution, and finally magnetically stir the electrolyte for 3 minutes to obtain a well-mixed electrolyte system. The voltage of anodizing is 35V, and the time is 6h. After oxidation, the Ti sheet was rinsed with deionized water and drie...
Embodiment 3
[0035] (1) Pretreatment: Cut out a Ti sheet with a size of 1cm*4cm with scissors, ultrasonically clean it with ethanol, acetone, and deionized water in sequence to remove surface impurities, and dry it for later use;
[0036] (2) Anodic oxidation: the titanium sheet obtained in step (1) is used as the anode, the titanium sheet is used as the negative electrode, and the dimethyl sulfoxide solution containing hydrogen fluoride is used as the electrolyte to carry out anodic oxidation, and the mass fraction of hydrogen fluoride in the electrolyte is 2%. When preparing the electrolyte, first measure the dimethyl sulfoxide solution and pour it into a small plastic beaker, then measure the hydrogen fluoride solution and add it to the dimethyl sulfoxide solution, and finally magnetically stir the electrolyte for 3 minutes to obtain a well-mixed electrolyte system. The voltage of anodizing is 40V, and the time is 8h. After oxidation, the Ti sheet was rinsed with deionized water and dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com