Method for increasing yield of 5-sulfoisophthalic acid for synthesis of ternary monomer
A technology of isophthalic acid and its production method, which is applied in the field of synthesis of intermediate isophthalic acid-5-sulfonic acid, can solve the problems of unreasonable ratio of raw materials, high cost of three monomers, long reaction time, etc., and achieve The effects of reducing sulfur trioxide loss, reducing process consumption, and improving operational safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
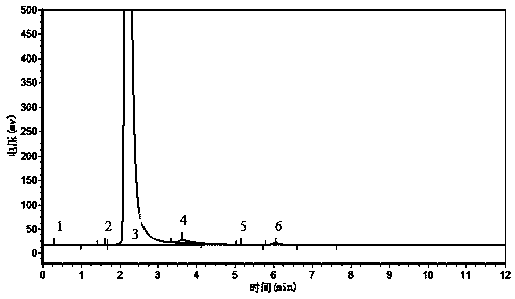
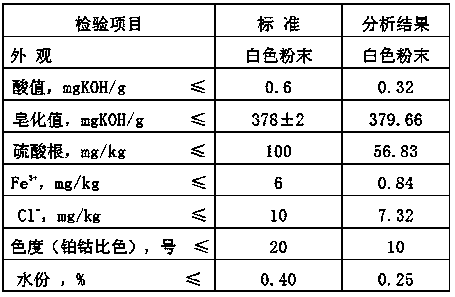
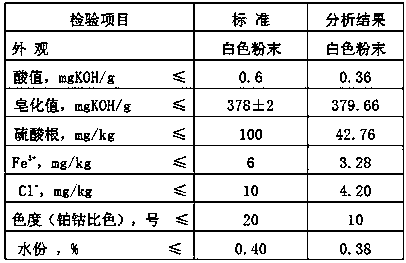
Image
Examples
Embodiment 1
[0070] Embodiment 1 A kind of production method that improves three monomer synthesis isophthalic acid-5-sulfonic acid yields
[0071] Include the following steps:
[0072] (1) Feeding
[0073] Material ratio: 1900 kg of isophthalic acid; 1620 kg of 65% oleum; the reaction is carried out in a 3000L glass-lined kettle;
[0074] Measure 1620 kg of fuming sulfuric acid into the high-level tank, put 1300 kg of fuming sulfuric acid into the sulfonation tank, and start stirring; add 1900 kg of isophthalic acid into the sulfonation tank within 60 minutes.
[0075] (2) filled with nitrogen
[0076] After the addition of isophthalic acid is completed, nitrogen is charged into the sulfonation tank. When the nitrogen pressure reaches 0.01MPa, the nitrogen addition valve is closed and the temperature rises.
[0077] (3) The first stage heating reaction
[0078] Within 40 minutes, the temperature of the sulfonation kettle was raised from normal temperature to 120° C., and the reaction ...
Embodiment 2
[0091] Embodiment 2 A kind of production method that improves three monomer synthesis isophthalic acid-5-sulfonic acid yields
[0092] Include the following steps:
[0093] (1) Feeding
[0094] Ratio of materials: 1925 kg of isophthalic acid; 1645 kg of 65% oleum; the reaction is carried out in a 3000L glass-lined kettle.
[0095] Measure 1645 kg of fuming sulfuric acid into the high-level tank, put 1320 kg of fuming sulfuric acid into the sulfonation tank, and start stirring; add 1925 kg of isophthalic acid into the sulfonation tank within 50 minutes.
[0096] (2) filled with nitrogen
[0097] After the addition of isophthalic acid is completed, nitrogen is charged into the sulfonation tank. When the nitrogen pressure reaches 0.01MPa, the nitrogen addition valve is closed and the temperature rises.
[0098] (3) The first stage heating reaction
[0099] In the first stage, the temperature of the sulfonation tank was raised from normal temperature to 122°C within 50 minutes...
Embodiment 3
[0112] Embodiment 3 A kind of production method that improves three monomer synthesis isophthalic acid-5-sulfonic acid yields
[0113] Include the following steps:
[0114] (1) Feeding
[0115] Ratio of materials: 1950 kg of isophthalic acid; 1660 kg of 65% oleum; the reaction is carried out in a 3000L glass-lined kettle.
[0116] Measure 1660 kg of fuming sulfuric acid into the high-level tank, put 1330 kg of fuming sulfuric acid into the sulfonation tank, and start stirring; add 1950 kg of isophthalic acid into the sulfonation tank within 60 minutes.
[0117] (2) filled with nitrogen
[0118] After the addition of isophthalic acid is completed, nitrogen is charged into the sulfonation kettle. When the nitrogen pressure reaches 0.012MPa, the nitrogen addition valve is closed to start heating.
[0119] (3) The first stage heating reaction
[0120] In the first stage, the temperature of the sulfonation tank was raised from normal temperature to 124° C. within 60 minutes, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com