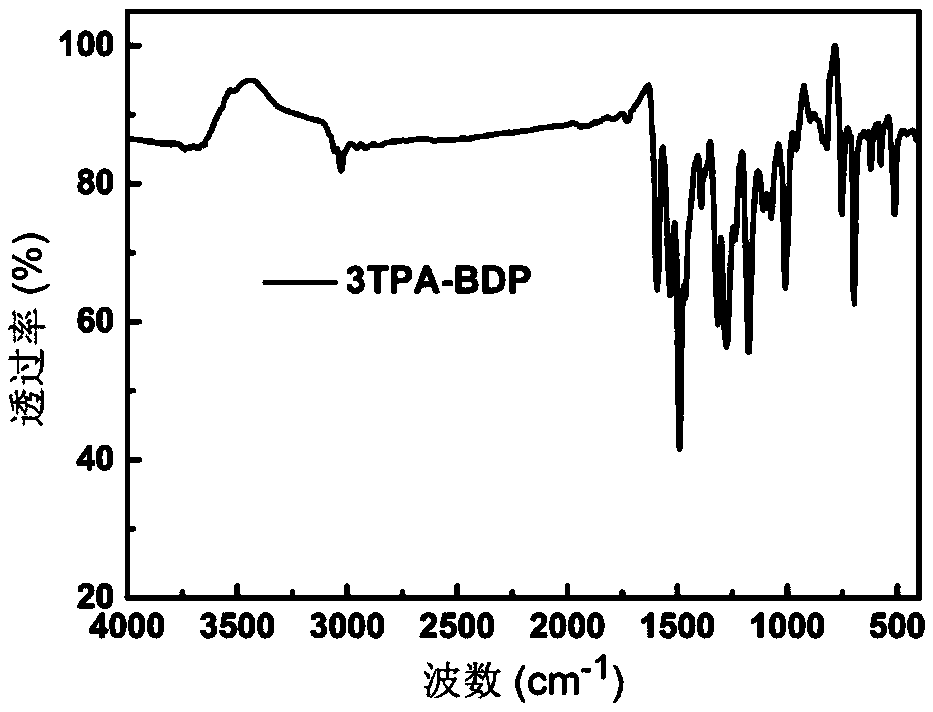
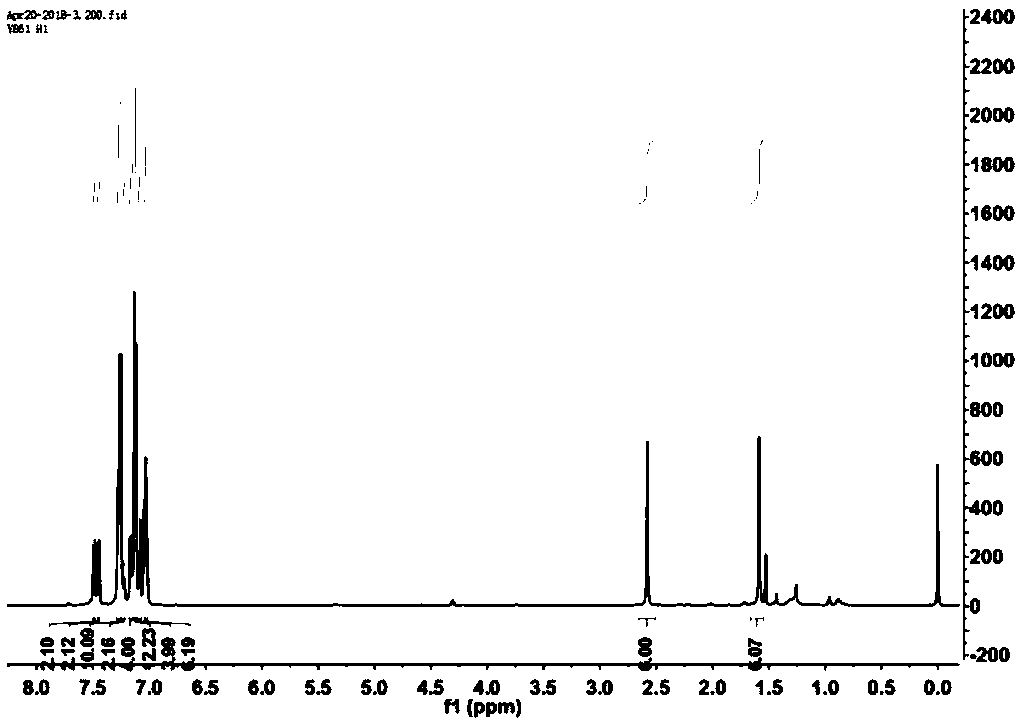
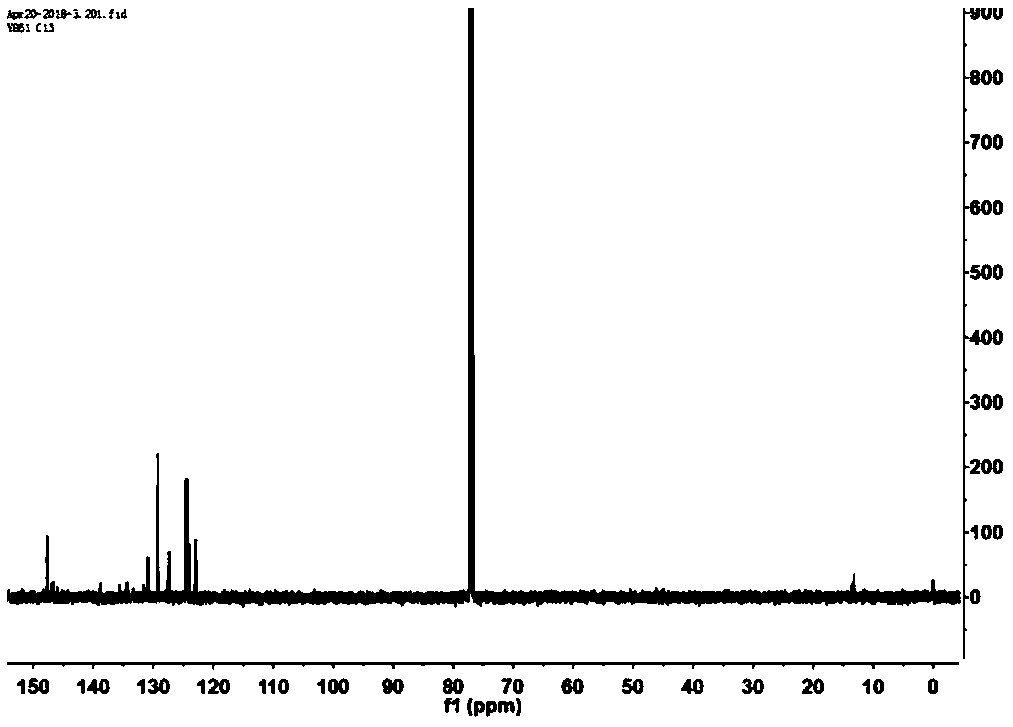
Boron-dipyrromethene derivative and nano-particle, preparation methods thereof, and applications of nano-particle
A dipyrromethene, nanoparticle technology, applied in the field of boron-dipyrromethene derivatives, preparation, and nanoparticles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The present invention also provides a method for preparing boron-dipyrromethene derivatives, the method comprising:
[0043] Step 1: Dissolve 4-formyltriphenylamine and 2,4-dimethylpyrrole in a dichloromethane solution in a reaction vessel, add trifluoroacetic acid, and stir in the dark under nitrogen protection. The stirring temperature is preferably Room temperature, the stirring time is preferably 20-28h, more preferably 24h, then add chlorobenzoquinone to the solution to continue stirring, the stirring time is preferably 50-60min, then add triethylamine dropwise, the dropwise Add time is preferably 10-20min, more preferably 15min, continue to stir, said stirring time is preferably 10-20min, more preferably 15min, then add boron trifluoride diethyl ether drop by drop, said dropping time is preferably 10-20min, more preferably 15min, continue stirring, the stirring time is preferably 1-3h, more preferably 2h, after the reaction is finished, it is preferably dried over...
Embodiment 1
[0056] In a 250mL three-necked flask, 4-formyltriphenylamine (0.46g, 1.7mmol) and 2,4-dimethylpyrrole (0.35g, 3.7mmol) were dissolved in 150mL of dichloromethane solution, and 1 drop of ( 0.05mL) trifluoroacetic acid, under the protection of nitrogen, stir at room temperature for 24 hours in the dark, then add chlorobenzoquinone (0.42g, 1.7mmol) to the solution, continue to stir for 1 hour, then slowly add 5mL trichloroquinone dropwise within 15 minutes Ethylamine, and then continue to stir for 15 minutes, then slowly add 5 mL of boron trifluoride diethyl ether dropwise within 15 minutes, and continue to stir for 2 hours. After the reaction, extract with water, add anhydrous sodium sulfate to dry, filter, remove the solvent under reduced pressure, and separate and purify by column chromatography to obtain 1,3,5,7-tetramethyl-8-triphenylamino-BODIPY as a yellow solid.
[0057] Dissolve iodic acid (0.35g, 2mmol) in a small amount of water, then slowly add a solution of 1,3,5,7-t...
Embodiment 2
[0061] In a 250mL three-necked flask, 4-formyltriphenylamine (0.46g, 1.7mmol) and 2,4-dimethylpyrrole (0.35g, 3.7mmol) were dissolved in 150mL of dichloromethane solution, and 1 drop of ( 0.05mL) trifluoroacetic acid, under the protection of nitrogen, stir at room temperature for 24 hours in the dark, then add chlorobenzoquinone (0.42g, 1.7mmol) to the solution, continue to stir for 1 hour, then slowly add 5mL trichloroquinone dropwise within 15 minutes Ethylamine, and then continue to stir for 15 minutes, then slowly add 5 mL of boron trifluoride diethyl ether dropwise within 15 minutes, and continue to stir for 2 hours. After the reaction, extract with water, add anhydrous sodium sulfate to dry, filter, remove the solvent under reduced pressure, and separate and purify by column chromatography to obtain 1,3,5,7-tetramethyl-8-triphenylamino-BODIPY as a yellow solid.
[0062] Dissolve iodic acid (0.35g, 2mmol) in a small amount of water, then slowly add a solution of 1,3,5,7-t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Relative molecular mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com