Novel D-A-D organic near-infrared tumor therapy reagent based on diketopyrrolopyrrole and preparation method thereof
A technology for diketopyrrolopyrrole, tumor treatment, applied in diketopyrrolopyrrole dyes, organic chemistry, antitumor drugs, etc., can solve the problems of difficult modification, restricted material development, poor photostability, etc. Small side effects, excellent photothermal conversion ability and singlet oxygen generation ability, and clear reagent structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The preparation of embodiment 1PD-Br
[0053] In a two-neck flask (250mL), dissolve 10-(4-bromophenyl)-10H-phenothiazine (3.540g, 10mmol) in 30mL of chloroform, replace nitrogen three times, add N,N-dimethyl Add formamide (7mL, 88.00mmol), add phosphorus oxychloride (7.8mL, 80.00mmol), heat to 90°C, and stir for 12 hours. Pour into ice water, adjust the pH to 6-7 with sodium hydroxide, extract with dichloromethane, dry, concentrate, and separate by column chromatography to obtain yellow powder 10-(4-bromophenyl)-10H-phenothiazine-3- Formaldehyde 2.9g, yield 75%. 1 H NMR (400MHz, CDCl 3 ): δppm7.70 (d, J=8.4Hz, 2H), 7.26 (d, J=8.4Hz, 2H), 7.04 (dd, J 1 =7.4,J 2 =1.8Hz, 2H), 6.88(m, 4H), 6.24(dd, J 1 =8.0,J 2 = 1.4Hz, 2H).
[0054] In a two-necked flask (250 mL), 1,8-diazabicycloundecene-7-ene (2 mL, 6.24 mmol) and lithium chloride (0.308 g, 7.28 mmol) were dissolved in tetrahydrofuran (50 mL), Replace nitrogen three times, cool down to 0°C, add ethyl 2-(diethoxyp...
Embodiment 2
[0058] Embodiment 2PD-Br NPs preparation
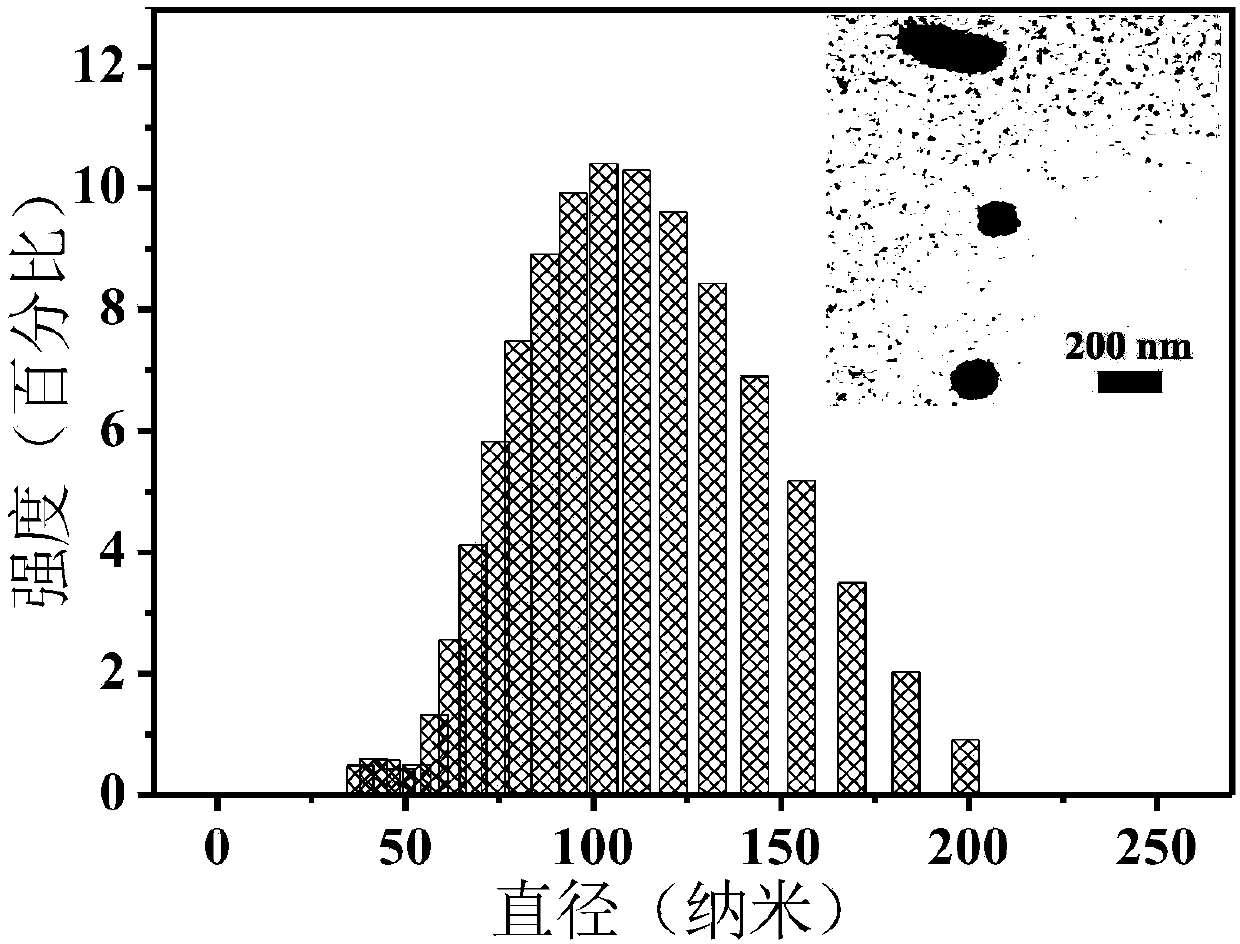
[0059] Dissolve PD-Br (0.002g) in 100 μL THF, slowly add dropwise (drop rate: 15-20 drops / min) into 10 mL PBS with rapid stirring (1000 rpm), stir for 5 minutes, blow nitrogen to remove THF , to obtain organic nanoparticles PD-Br NPs. figure 1 It is the dynamic light scattering (DLS) result and the transmission electron microscope (TEM) picture of the organic nanoparticle PD-Br NPs test. The particle size of the obtained nanoparticles is about 100nm.
Embodiment 3
[0060] Embodiment 3PD-Br NPs spectrum test:
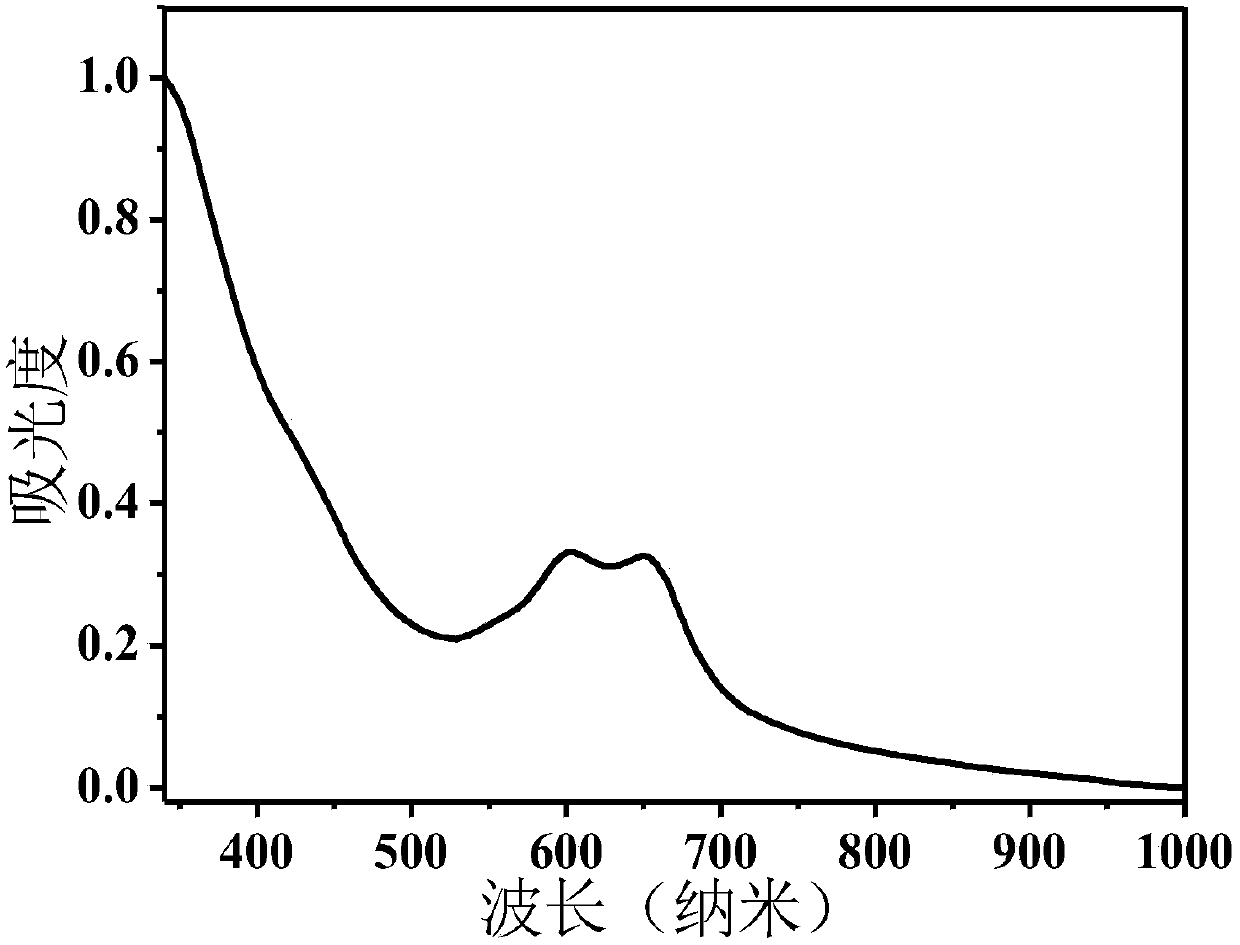
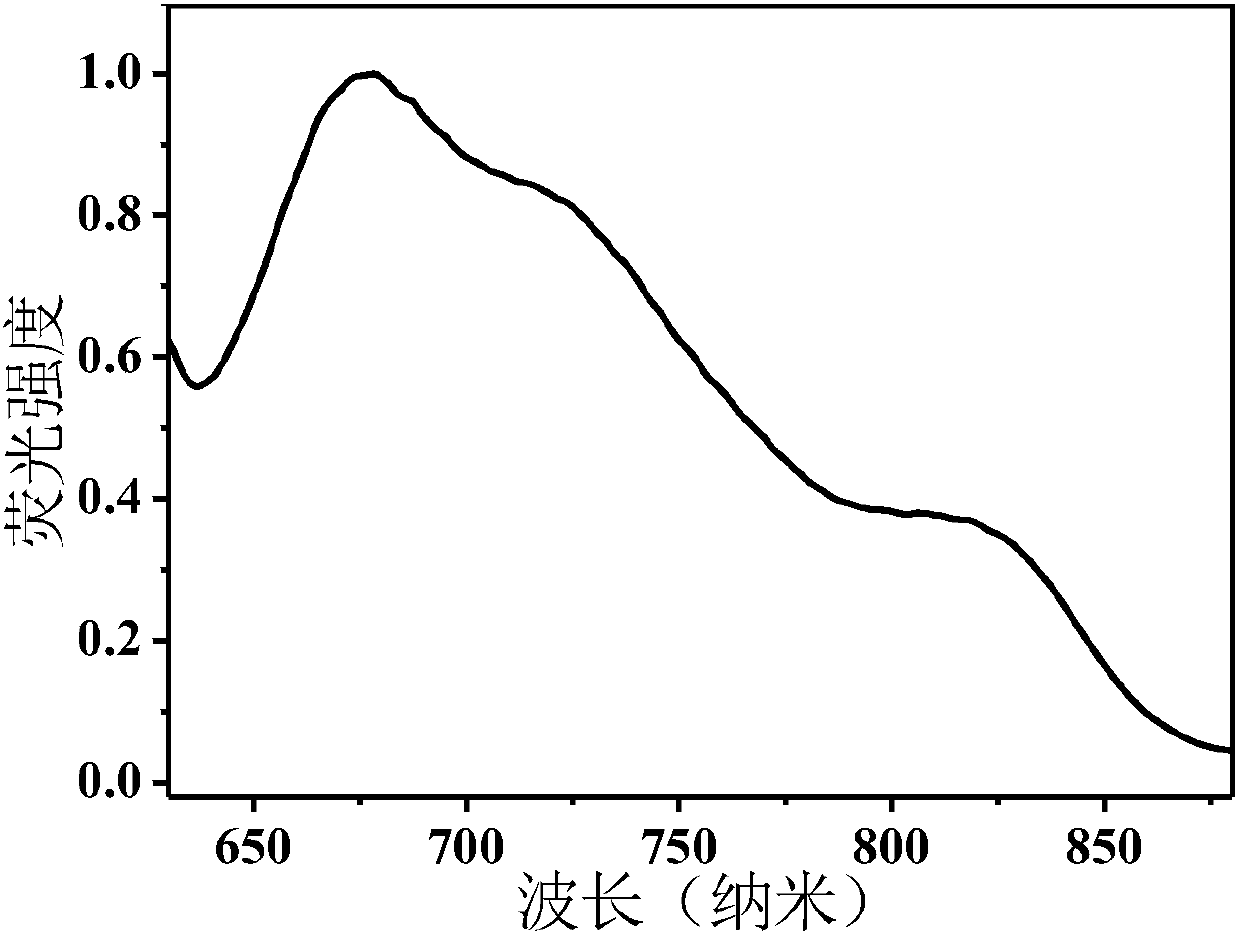
[0061] Select a 1 cm wide quartz cuvette, add 3 mL of PD-Br NPs PBS (50 μg / mL) solution, and test its ultraviolet absorption spectrum and fluorescence emission spectrum. Such as figure 2 As shown, in the ultraviolet-visible light absorption spectrum of PD-Br NPs, there are absorption peaks in the range of 600-1000nm, the maximum absorption peak is at 675nm, and the spectral range reaches the near-infrared region. image 3 It is the fluorescence emission spectrum of PD-Br NPs, and its emission spectrum ranges from 600-900nm, reaching the near-infrared region.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com