Preparation of pig secretory IgA secretory component and purification and renaturation method of pig secretory IgA secretory component
A secreted, positive technology, applied in the field of recombinant proteins, can solve the problems of low SC, high cost, cumbersome SC steps, etc., and achieve the effects of simple operation, increased concentration, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
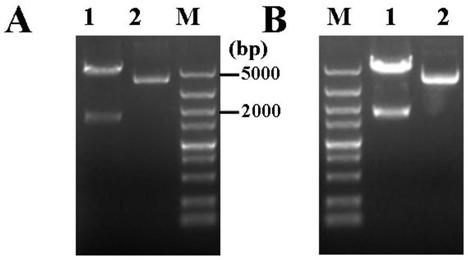
[0040] Construction of recombinant plasmid for prokaryotic expression of SC protein
[0041] 1. Primer Design
[0042] Download the gene sequence of wild boar (Sus scrofa) polymeric immunoglobulin receptor (PIGR) from the NCBI website, and use bioinformatics software to analyze the signal peptide, transmembrane region and antigenicity of the protein. , designed a large number of primers for PCR amplification of SC gene, after a large number of screening, a set of primers SC-F and SC-R with strong sensitivity and specificity were selected, and BamHI and SalI were introduced into primers SC-F and SC-R respectively Restriction site, the sequence of the primer is as follows:
[0043] SC-F:5'-CCG GAATTC TCGGTGTCCATCAGATGCTACTA-3' (SEQ ID NO: 1);
[0044] SC-R:5'-ACGC GTC GAC CTGCAGGTACTCCACTCCCACTA-3' (SEQ ID NO: 2).
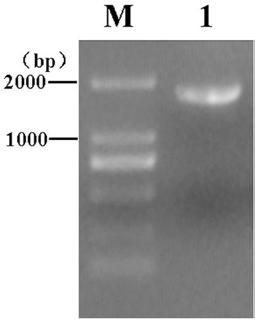
[0045] 2. Amplification of SC gene fragments
[0046] According to the instructions of the RNA extraction kit, the RNA of the porcine small intestine tissue w...
Embodiment 2
[0053] Fusion expression of SC protein
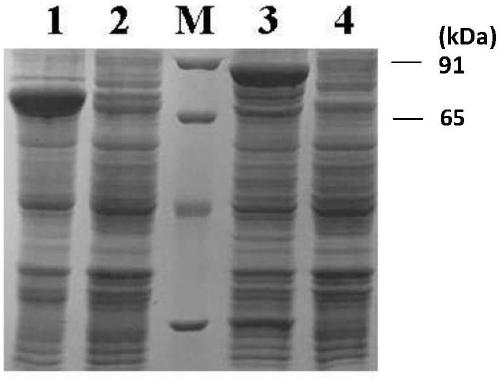
[0054] Method: The recombinant plasmids pET28a-SC and pGEX-4T-1-SC were respectively transformed into the expression strain BL21(DE3), and a single colony was picked in LB liquid medium. Detect the OD260 value in the LB liquid medium. If the OD260 is 0.4-0.6, add IPTG with a final concentration of 1mmol / L to induce. The induction condition is 16°C for 20 hours. identification of recombinant proteins.
[0055] Result: find that the protein expression of pET28a-SC (swimming lane 1) is higher than the protein expression of pGEX-4T-1-SC (swimming lane 3) ( image 3 ), the protein expression level was 10-20% higher; then the pET28a-SC plasmid with higher expression level was transformed into BL21(DE3) and Rosetta host bacteria respectively, and it was found that the protein expression level in Rosetta host bacteria (lane 3) was higher than BL21(DE3) host bacteria (lane 1), the protein expression level is 10-20% higher ( Figure 4 ).
[0...
Embodiment 3
[0058] Purification and renaturation of SC protein
[0059] 1. Purification of SC protein
[0060] The Rosetta host bacterium after the expression of the pET28a-SC plasmid of Example 2 was induced and expressed was washed 2-3 times with PBS, and the bacterial cells were broken by an ultra-high pressure continuous flow cell disruptor, centrifuged at 10000rpm for 15min, and the supernatant and the precipitate were separated, and SDS-PAGE was used to identify the recombinant protein SC mainly in the form of inclusion body. After the inclusion body is washed 3 times with the washing solution prepared by the present invention, most of the foreign proteins of the bacteria can be removed, which is better than that of the washing solution of the control group. Then the inclusion body protein was dissolved with 8M urea, and further purified by Ni column, it was found that there were almost no impurities and the bands were clearly visible, and the purification rate reached 90%, indicat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com