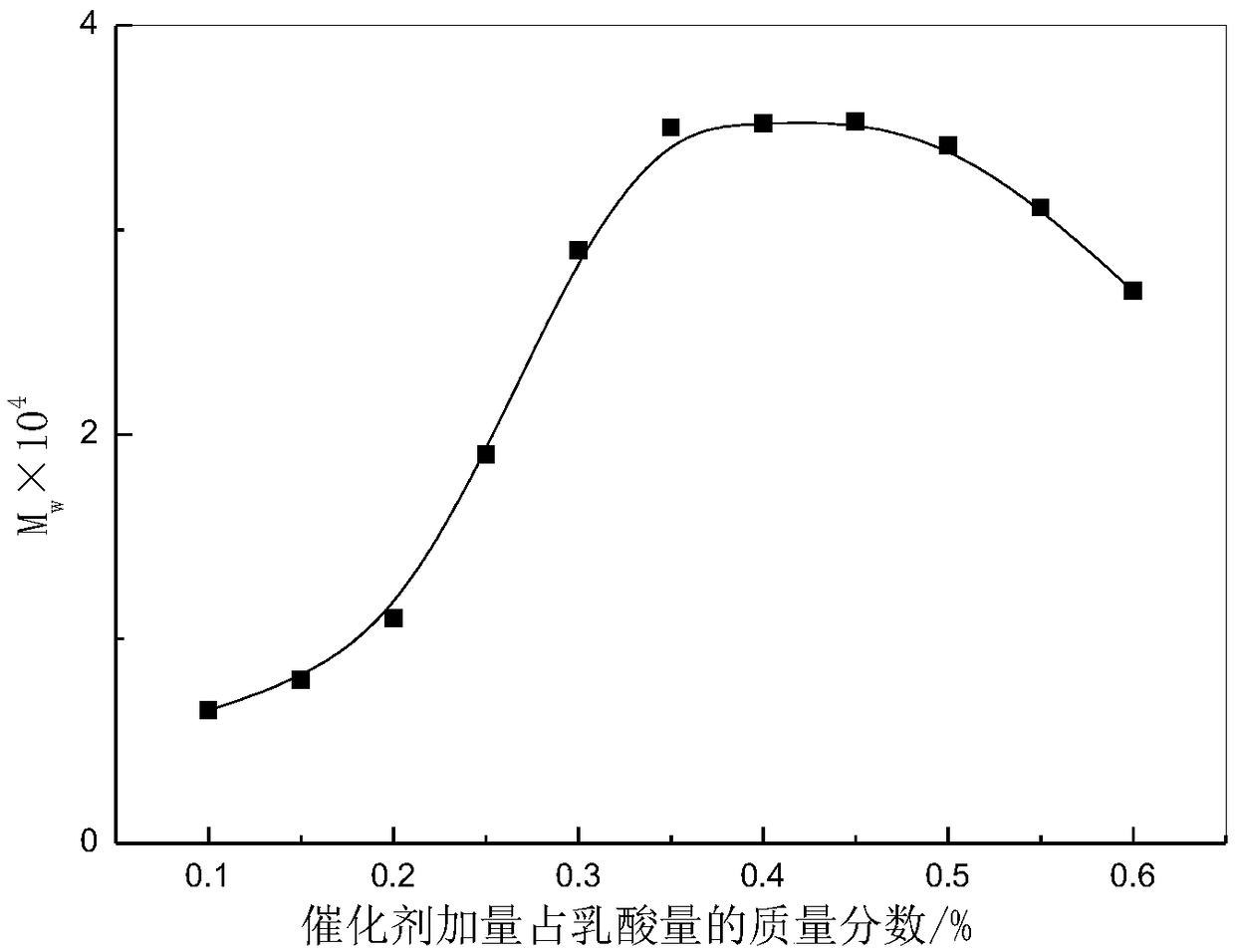
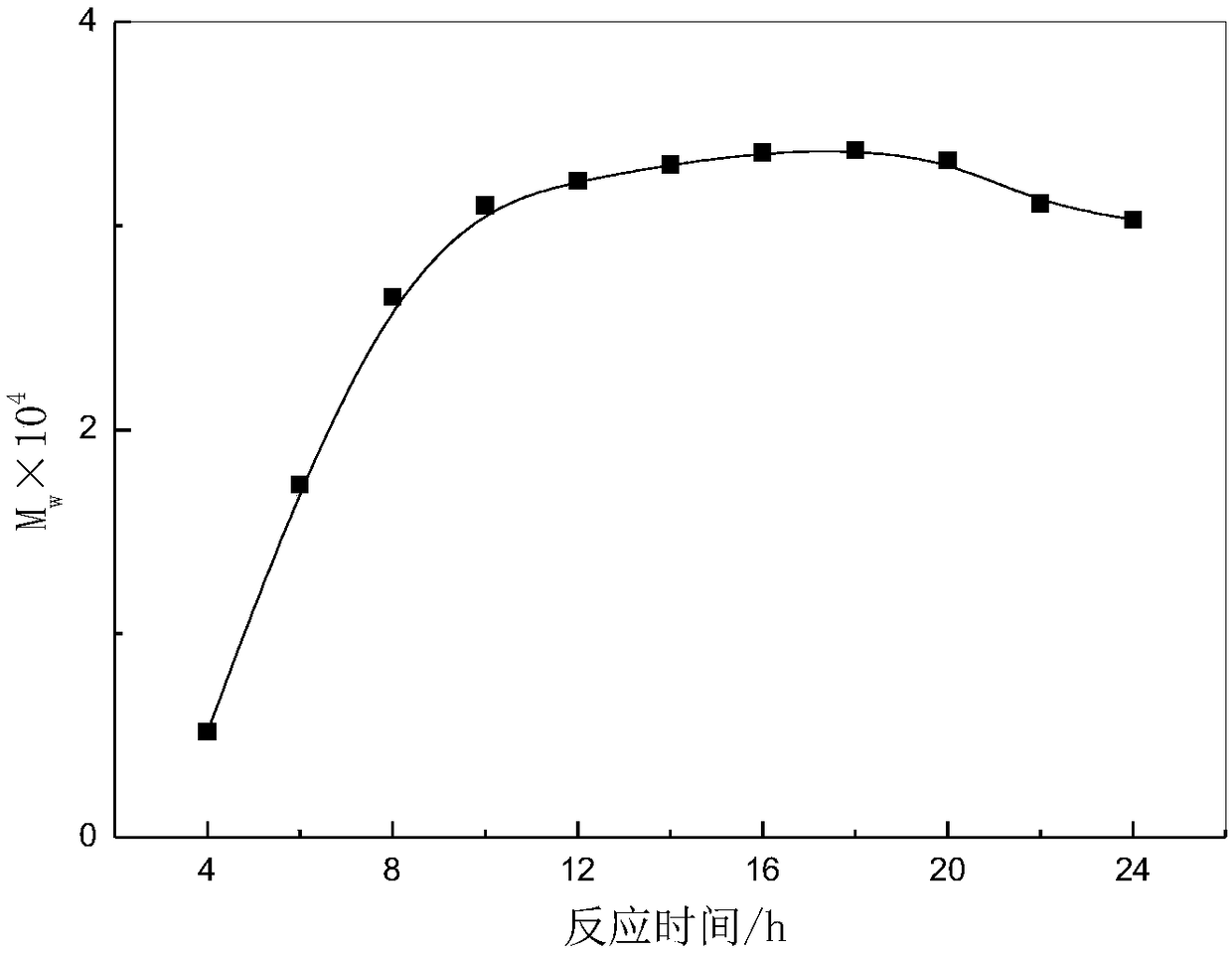
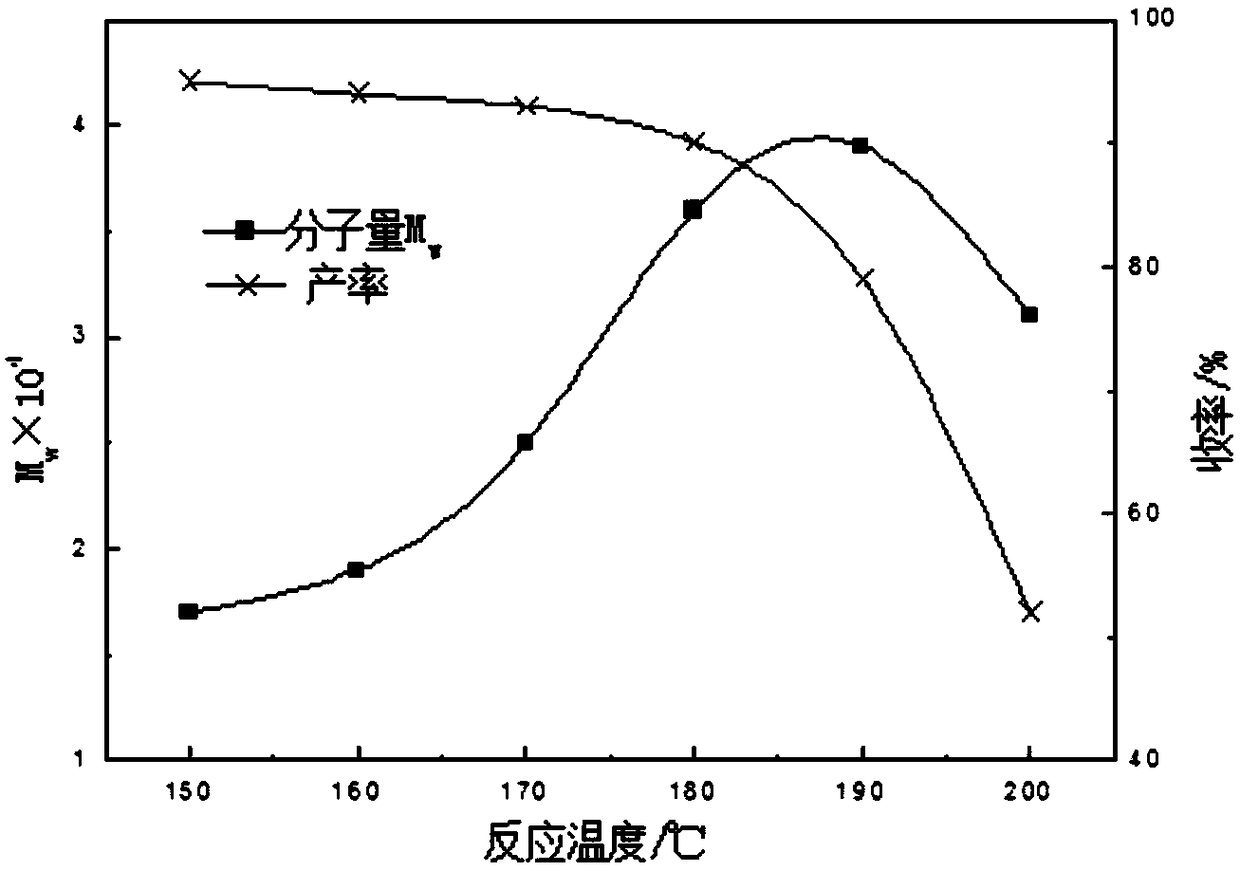
Method used for producing poly L-lactic acid from L-lactic acid through oligomerization, melt phase polycondensation, and solid phase polycondensation
A technology of melt polycondensation and solid-state polycondensation, which is applied in the field of deep processing of lactic acid, can solve problems such as high cost, unsuitable for industrial application, and toxicity, and achieve mild reaction conditions, enhance product competitiveness, and reduce costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] A method utilizing L-lactic acid to produce poly-L-lactic acid through oligomerization, melt polycondensation and solid phase polycondensation, comprising the steps of:
[0109] (1) Raw material preparation: 45wt% L-lactic acid solution produced by the applicant, the optical purity of L-lactic acid is greater than or equal to 99.5%;
[0110] (2) Dehydration oligomerization to obtain L-lactic acid oligomers;
[0111] Add ethyl pyruvate to the L-lactic acid solution and stir to obtain a mixed solution, the volume ratio of ethyl pyruvate to the L-lactic acid solution is 1:1;
[0112] Simultaneously add cobalt oxide and two-cysteine methyl ester vanadyl as catalyst in mixed solution according to the following steps:
[0113] During the initial reaction (0h): the mass ratio of cobalt oxide and di-cysteine methyl ester vanadyl is 1:1.5, and the amount of cobalt oxide added during the initial reaction is 1 / 2 of the total amount of cobalt oxide added; The reaction tempera...
Embodiment 2
[0119] A method utilizing L-lactic acid to produce poly-L-lactic acid through oligomerization, melt polycondensation and solid phase polycondensation, comprising the steps of:
[0120] (1) Raw material preparation: 60wt% L-lactic acid solution produced by the applicant, the optical purity of L-lactic acid is greater than or equal to 99.5%;
[0121] (2) Dehydration oligomerization to obtain L-lactic acid oligomers;
[0122] Add ethyl pyruvate to L-lactic acid solution and stir to obtain a mixed solution, the volume ratio of ethyl pyruvate to L-lactic acid solution is 1:1; add cobalt oxide and di-cysteine methyl ester to the mixed solution Vanadyl is used as a catalyst; the amount of cobalt oxide used is 0.04% of the mass of L-lactic acid, the amount of di-cysteine methyl ester vanadyl is 0.08% of the mass of L-lactic acid, and the reaction is stirred for 3 hours at room temperature; The preparation method of vanadyl methyl ester is as follows: methyl cysteine and vanadyl...
Embodiment 3
[0125] A method utilizing L-lactic acid to produce poly-L-lactic acid through oligomerization, melt polycondensation and solid phase polycondensation, comprising the steps of:
[0126] (1) Raw material preparation: 50wt% L-lactic acid solution produced by the applicant, the optical purity of L-lactic acid is greater than or equal to 99.5%;
[0127] (2) Dehydration oligomerization to obtain L-lactic acid oligomers;
[0128] Add ethyl pyruvate to the L-lactic acid solution and stir to obtain a mixed solution, the volume ratio of ethyl pyruvate to the L-lactic acid solution is 1:1;
[0129] Simultaneously add cobalt oxide and two-cysteine methyl ester vanadyl as catalyst in mixed solution according to the following steps:
[0130] During the initial reaction (0h): the mass ratio of cobalt oxide and di-cysteine methyl ester vanadyl is 1:1.5, and the amount of cobalt oxide added during the initial reaction is 1 / 2 of the total amount of cobalt oxide added; The reaction tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com