Preparation method for metal oxide nano-materials, and method for separating boron isotopes by using nano-materials as stationary phase of simulated moving bed
A technology for simulating moving bed and nanomaterials, applied in the field of boron isotope separation by metal oxide nanomaterials, can solve the unsatisfactory and limited industrial application of chromatography, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
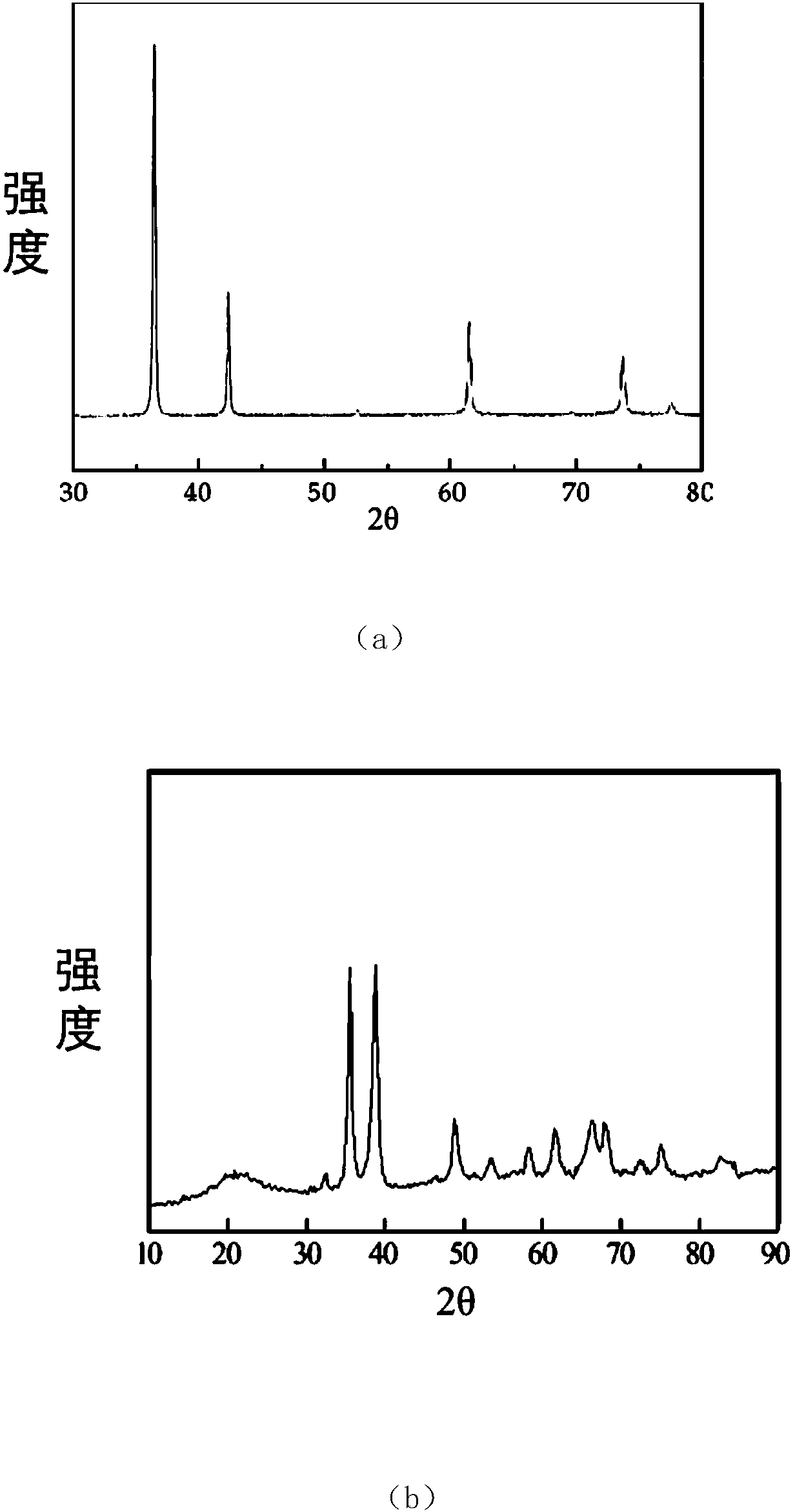
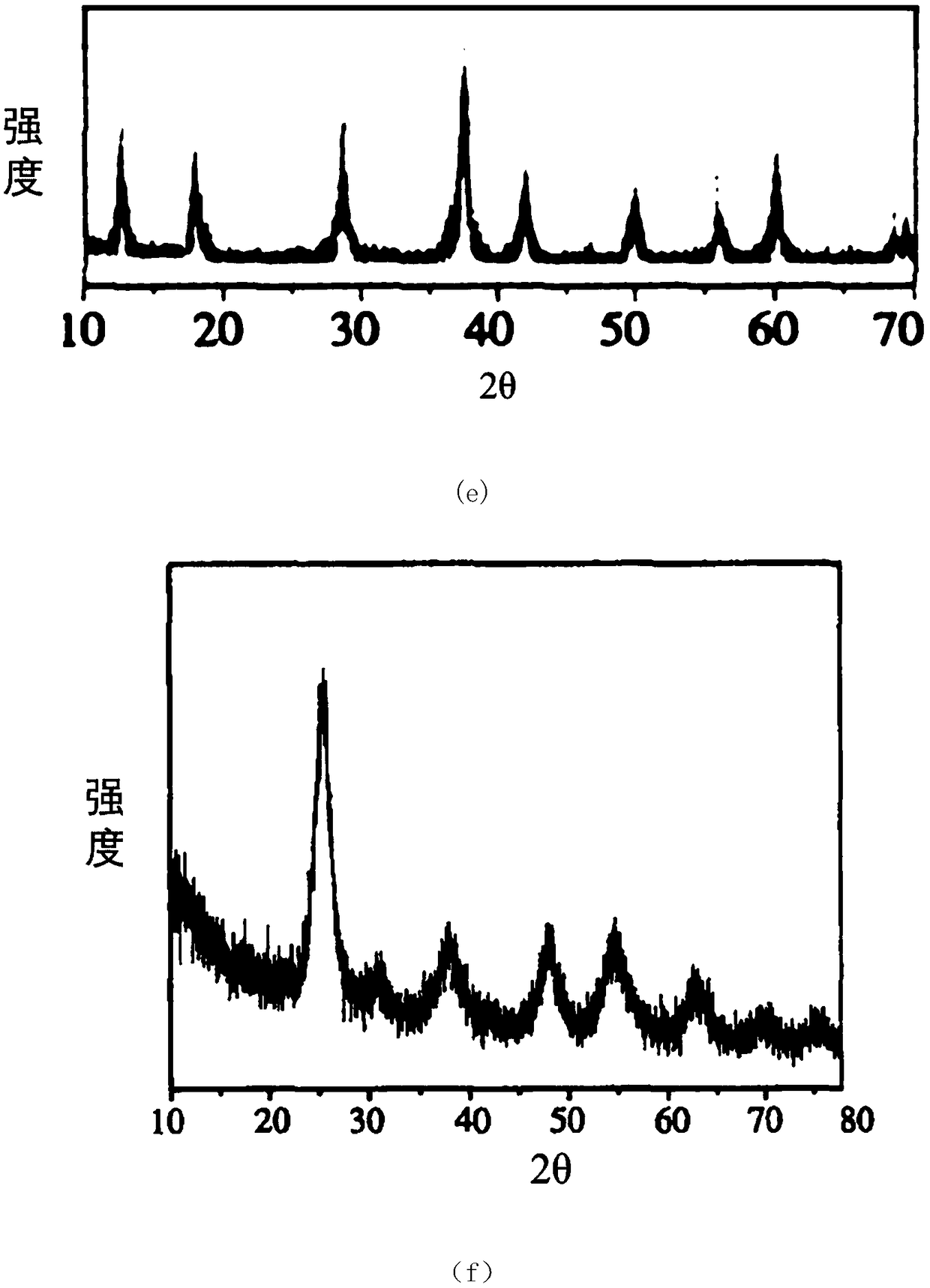
[0064] 1. Preparation of metal oxide nanomaterial Cu 2 o
[0065] The reaction was carried out in a 2L three-necked flask, and 800mL, 1.0mol·L-1 NaOH solution and 40mL, 1.0mol·L-1 C 6 h 12 o 6 ·H 2 O solution, added to an electrically stirred 200 mL, 20 wt% CuSO4 5H at a certain initial temperature 2 In the O solution, there are blue flocs in the solution at this time, the oil bath is heated to 70°C, and the blue flocs
[0066] Disappeared, brick-red or brick-yellow particles precipitated in the system, the resulting product was filtered, and the filter cake was washed with hot distilled water and absolute ethanol for 3 to 4 times, and finally the obtained sample was vacuum-dried at 60°C for 2 hours to obtain powdered Cu2O sample.
Embodiment 2
[0068] Preparation of Metal Oxide Nanomaterial CuO
[0069] 0.5molCu(NO 3 ) 2 ·3H 2 O was dissolved in 1000 mL of absolute ethanol, and magnetically stirred to dissolve it, and Cu(NO 3 ) 2 ethanol solution. The solution is slowly added dropwise with concentrated ammonia water under stirring, and the pH value is controlled at about 9, so that it can completely react to form Cu(OH) 2 Sol. Cu(OH) 2 The sol was transferred to a high-pressure reactor, the temperature was programmed to 250 °C, and the pressure was controlled to 7.5 MPa. After a period of heat preservation and pressure, the gas was slowly released, and then the protective gas (N 2 ) was naturally cooled to room temperature, and black fluffy nano-sized CuO ultrafine powder was obtained.
Embodiment 3
[0071] Preparation of Metal Oxide Nanomaterial ZnO
[0072] A certain amount of 6gZn(NO 3 ) 2 ·6H 2 O was dissolved in 50ml of deionized water, and the aqueous solution of sodium hydroxide (4g / L) and sodium carbonate (pH=10) with a certain concentration prepared was slowly dripped successively under stirring until the pH was 9, and the resulting product was Zn 2 (OH) 2 CO 3 , filtered and washed, dried at about 70°C, ground and sieved, and then put into a muffle furnace for calcination to obtain nano-zinc oxide. The calcination temperature used was 500°C and the calcination time was 1 h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com