New green technology for preparation of 2-chloronicotinic acid
A technology for chloronicotinic acid and nicotinic acid is applied in the field of green synthesis of 2-chloronicotinic acid to achieve the effects of short process flow, corrosion avoidance and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
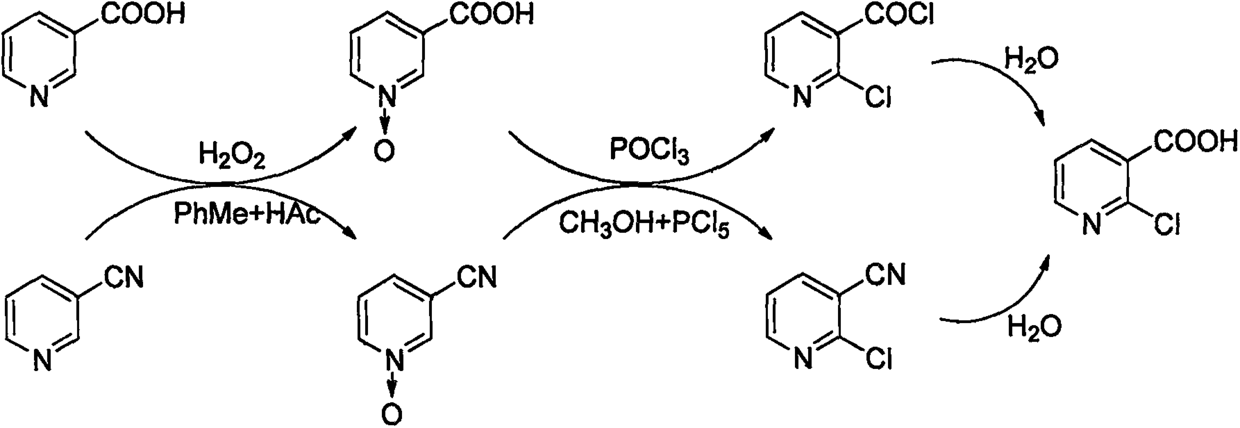
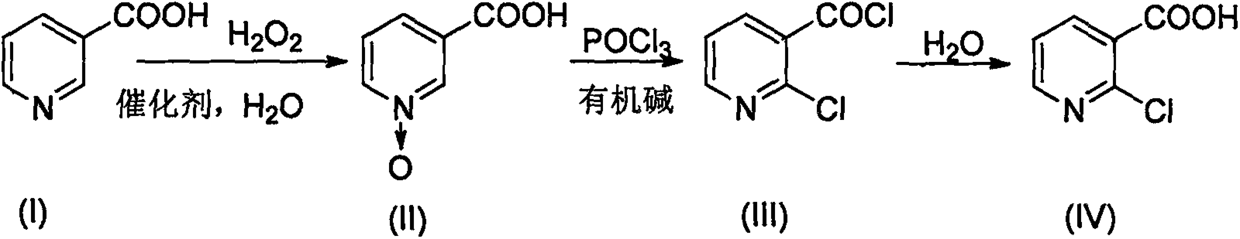
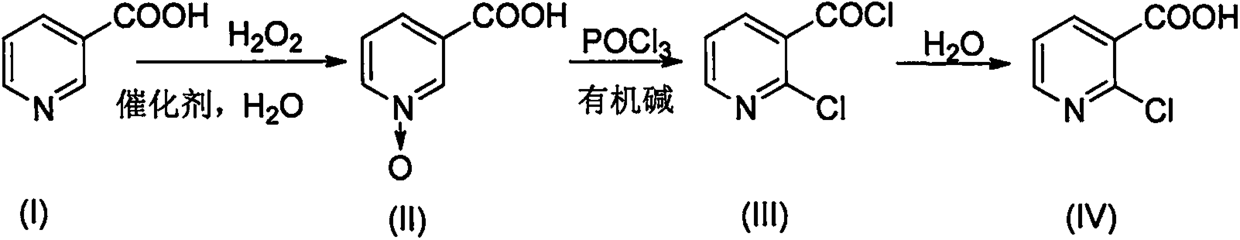
[0027] Add 100ml of water, 100g of nicotinic acid, and 0.66g of molybdenum acid to the three-necked flask in sequence, and raise the temperature to 92°C. After the temperature stabilizes, start to add 104ml of hydrogen peroxide dropwise for 3 hours at a constant speed. Niacin content < 0.5%, drop to 0-5°C after heat preservation, stir for 0.5h, filter, the mother liquor will be reused in the next batch of oxidation reaction, and the filter cake is vacuum-dried at 60°C to obtain 108.7g of white crystalline powder N-oxidized nicotinic acid, Yield 97.4% [HPLC content 99.8%, moisture 0.22%, mp 261.4-261.6°C (260-262°C in literature)].
[0028] Add 146ml of phosphorus oxychloride to the three-necked flask, stir in an ice bath and cool down to 10°C, start to slowly add 32g (0.23mol) of N-oxidized nicotinic acid, then add 36ml of triethylamine (0.26mol) dropwise at 15°C, and dropwise The temperature was raised to 100°C with a uniform gradient for 6 hours.
[0029] The above reaction...
Embodiment 2
[0031] Add 100ml of N-oxidized nicotinic acid mother liquor, 100g of nicotinic acid, and 0.66g of molybdic acid in sequence to the three-necked flask, raise the temperature to 92°C, and start adding 104ml of hydrogen peroxide dropwise after the temperature stabilizes, add dropwise for 3 hours at a constant speed, and keep warm at 95°C after the dropwise addition is completed 3h, TLC detects that the content of niacin is <0.5%. After the heat preservation, it is lowered to 0-5°C, stirred for 0.5h, filtered, and the mother liquor is recycled for the next batch of oxidation reaction. The filter cake is vacuum-dried at 60°C to obtain white crystalline powder N-oxide fume Acid 111.7g, yield 99.6% [HPLC content 99.8%, moisture 0.21%, mp 261.4-261.6°C (document 260-262°C)].
[0032] Add a total of 146ml of recovered phosphorus oxychloride and fresh phosphorus oxychloride to the three-necked flask, stir in an ice bath and cool down to 10°C, start to slowly add 32g of N-oxidized nicotin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com