Kilogram-grade preparation method of 3,3'-diamino-4,4'-azoxyfurazan
A technology of azofurazan oxide and diamino, which is applied in organic chemistry, offensive equipment, explosives processing equipment and other directions, can solve the problems of a large amount of acidic waste liquid, unsuitable process amplification, long reaction time, etc., and achieves optimized preparation process, reaction The effect of short time and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
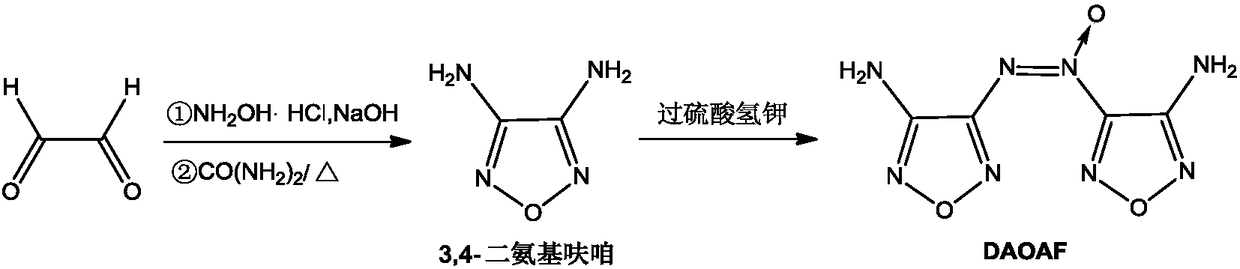
[0041] The kilogram-level preparation method of 3,3'-diamino-4,4'-azofurazan, the steps are:
[0042] a. Efficient preparation of intermediate 3,4-diaminofurazan:
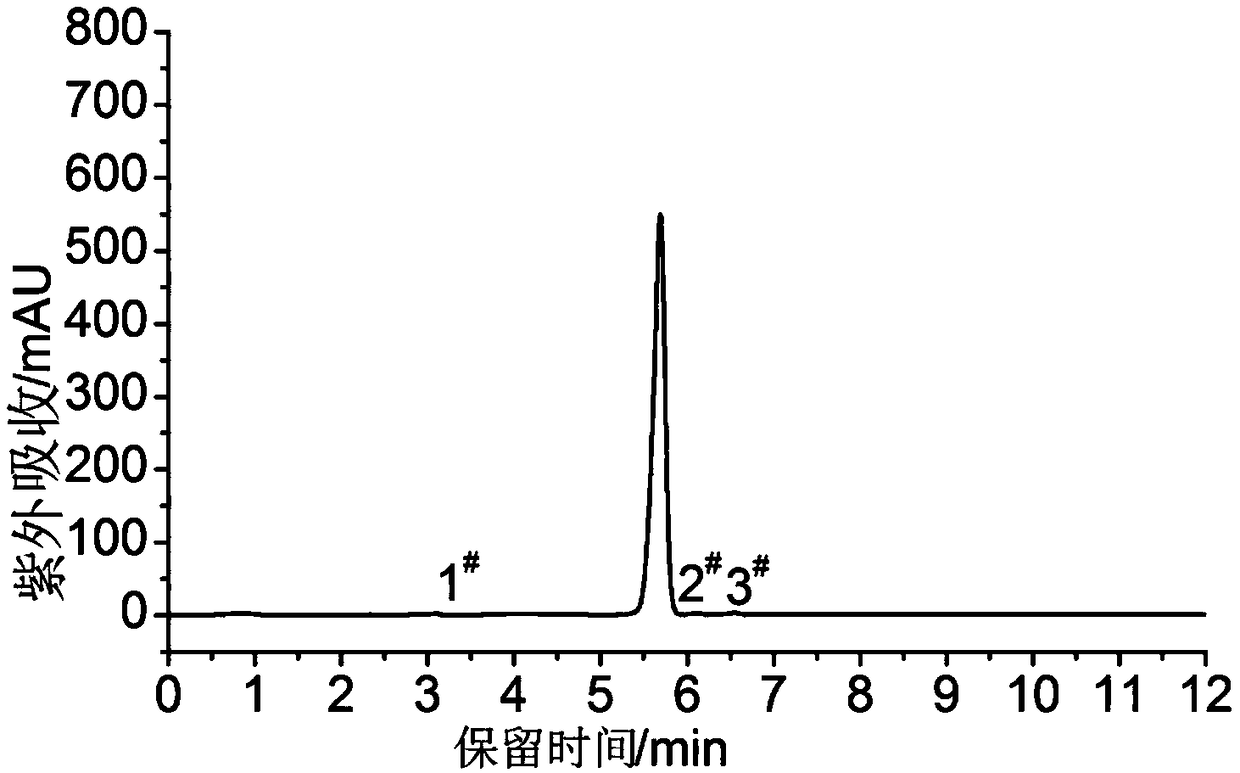
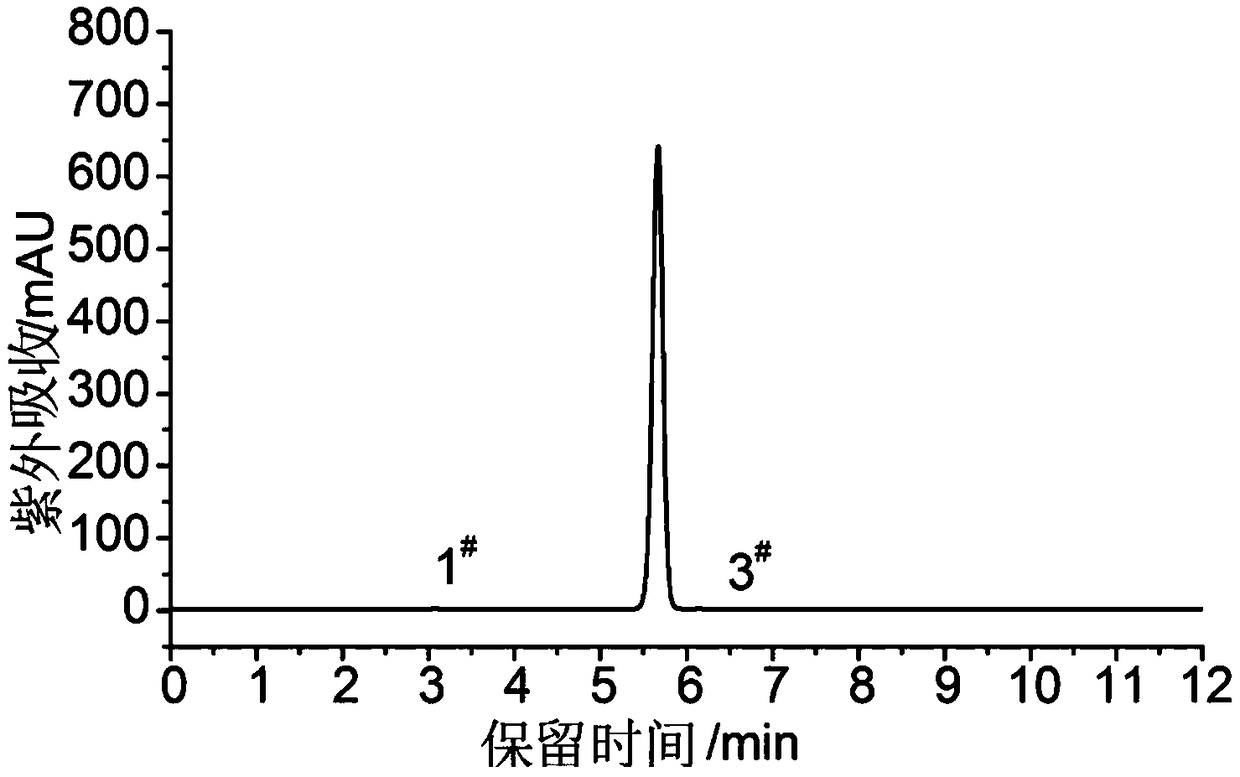
[0043] Under ice-bath temperature and stirring, add 1.0mol glyoxal into the reactor (three-necked flask), add 6.5mol hydroxylamine hydrochloride, and then slowly dropwise add 7.0mol sodium hydroxide solution in 600mL water to the reaction vessel. Add sodium hydroxide aqueous solution dropwise at a temperature <10°C; after dropping, raise the temperature to 100-108°C, reflux for 7 hours, add 5.8mol urea, and heat up to 100-110°C for reflux After reacting for 14 hours, after the reaction, the material was cooled (placed in a refrigerator) and kept in a refrigerator at 0-6°C, and white needle-like crystals were precipitated, namely the prepared 3,4-diaminofurazan, with a yield of 44% and a purity of 99.6% (HPLC area normalization method);
[0044] b. Efficient preparation of high-purity DAOAF:
[0045] Add 10.0 mol...
Embodiment 2
[0049] The kilogram-level preparation method of 3,3'-diamino-4,4'-azofurazan, the steps are:
[0050] a. Efficient preparation of intermediate 3,4-diaminofurazan:
[0051] Under ice-bath temperature and stirring, 115mL volume percent content is that 40% glyoxal aqueous solution is added in the reactor (three-necked flask), adds 450g hydroxylamine hydrochloride, then 280g sodium hydroxide is dissolved in the sodium hydroxide aqueous solution of 600mL water (Slowly) dropwise into the reactor (three-necked flask), and keep the temperature <10°C and dropwise add sodium hydroxide aqueous solution; after dropping, raise the temperature to 100-108°C, and reflux for 7h. Add 350g of urea, raise the temperature to 100-110°C for reflux reaction for 20 hours; after the reaction, the material is cooled (placed in the refrigerator) and refrigerated at 0-6°C, white needle-like crystals are precipitated, that is, the prepared 3,4-di Aminofurazan, yield 50%, purity 99.8% (HPLC area normalizat...
Embodiment 3
[0056] The kilogram-level preparation method of 3,3'-diamino-4,4'-azofurazan, the steps are:
[0057] a. Efficient preparation of intermediate 3,4-diaminofurazan:
[0058] Under ice-bath temperature and stirring, 115mL volume percent content is that 40% glyoxal aqueous solution is added in the reactor (three-necked flask), adds 450g hydroxylamine hydrochloride, then 280g sodium hydroxide is dissolved in the sodium hydroxide aqueous solution of 600mL water (Slowly) dropwise into the reactor (three-necked flask), and keep the temperature <10°C and dropwise add sodium hydroxide aqueous solution; after dropping, raise the temperature to 100-108°C, and reflux for 7h. 350g of urea was added, and the temperature was raised to 100-110°C for reflux reaction for 24h. After the reaction solution was cooled (placed in the refrigerator), it was refrigerated and left standing at 0-6°C, and white needle-like crystals were precipitated, namely the prepared 3,4-diaminofurazan, with a yield of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com