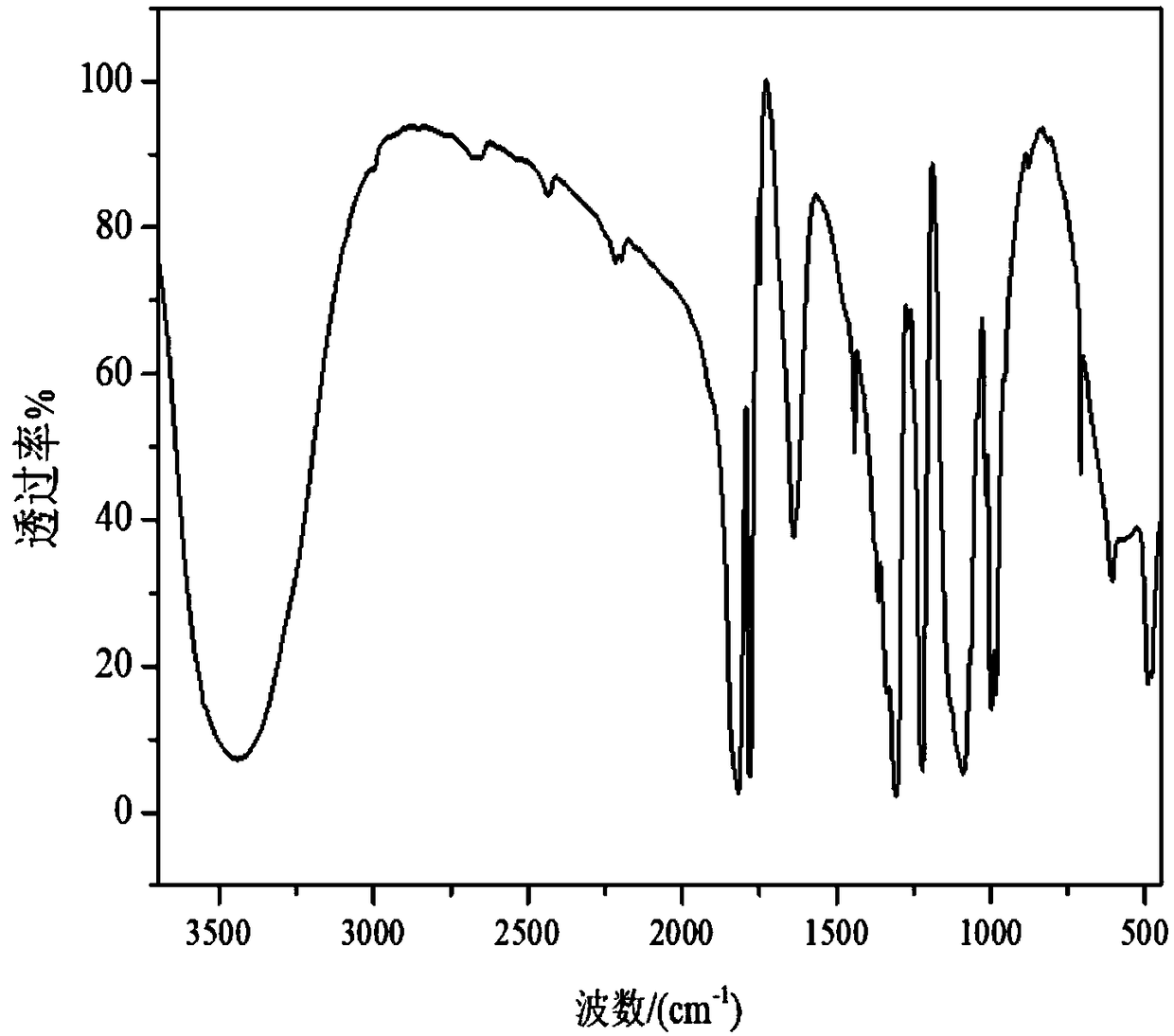
Lithium bis(oxalato)borate preparation method
A technology of lithium bis-oxalate borate and oxalic acid is applied in the field of preparation of lithium bis-oxalate borate, can solve the problems of harsh preparation conditions, high cost of raw materials, poor thermal stability, etc., achieves cheap and easy-to-obtain raw materials, simple and feasible process, and improved film formation. performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] A method for preparing lithium bis-oxalate borate, weighing each substance at a molar ratio of lithium hydroxide, oxalic acid and boric acid of 1:2:1, and then placing the lithium hydroxide and boric acid in an oven at 60°C for 4 hours Put the oxalic acid in an oven with a temperature of 120°C for 4 hours, then place the dried lithium hydroxide and oxalic acid in a ball mill for 15 minutes, and then add the dried boric acid to the ball mill for 15 minutes until a uniform The mixture was then dry-pressed into tablets under a pressure of 0.2 MPa, and then put into a container, first placed in a vacuum box with a temperature of 120°C for 6 hours, and then the temperature of the vacuum box was increased to 150°C so that the mixture was placed at a temperature of After 15 hours of reaction in a vacuum box at 150°C, a crude product of lithium bisoxalate borate is obtained. The crude product of lithium bisoxalate borate is then ground into a powder and fully dissolved in distill...
Embodiment 2
[0023] A method for preparing lithium bisoxalate borate, the steps of which are basically the same as those of the method for preparing lithium bisoxalate borate in Example 1, and the difference lies in:
[0024] 1. Lithium hydroxide and boric acid are placed in an oven at 50°C for 6 hours, and oxalic acid is placed in an oven at 130°C for 4.5 hours;
[0025] 2. The ball milling time of lithium hydroxide and oxalic acid is 25min, and the ball milling after boric acid is added is 25min;
[0026] 3. The mixture is dry pressed into tablets under a pressure of 0.22 MPa and then put into a container, first placed in a vacuum box with a temperature of 130°C for 5 hours, and then the temperature of the vacuum box is increased to 170°C so that the mixture is placed at 170°C Reaction in a vacuum box for 12 hours to obtain a crude product of lithium bisoxalate borate;
[0027] 4. Evaporate and concentrate the filtrate until the remaining liquid is 2% of the total filtrate;
[0028] 5. The produc...
Embodiment 3
[0031] A method for preparing lithium bisoxalate borate, the steps of which are basically the same as those of the method for preparing lithium bisoxalate borate in Example 1, and the difference lies in:
[0032] 1. Lithium hydroxide and boric acid are placed in an oven at 70℃ for 4.5 hours, and oxalic acid is placed in an oven at 105℃ for 6 hours;
[0033] 2. The ball milling time of lithium hydroxide and oxalic acid is 10 minutes, and the ball milling time after boric acid is added is 25 minutes;
[0034] 3. The mixture is dry pressed into tablets under a pressure of 0.25 MPa and then put into a container, first placed in a vacuum box with a temperature of 105°C for 8 hours, and then the temperature of the vacuum box is increased to 140°C so that the mixture is placed at a temperature of 140°C Reaction in a vacuum box for 24 hours to obtain a crude product of lithium bisoxalate borate;
[0035] 4. Evaporate and concentrate the filtrate until the remaining liquid is 1.5% of the total...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com