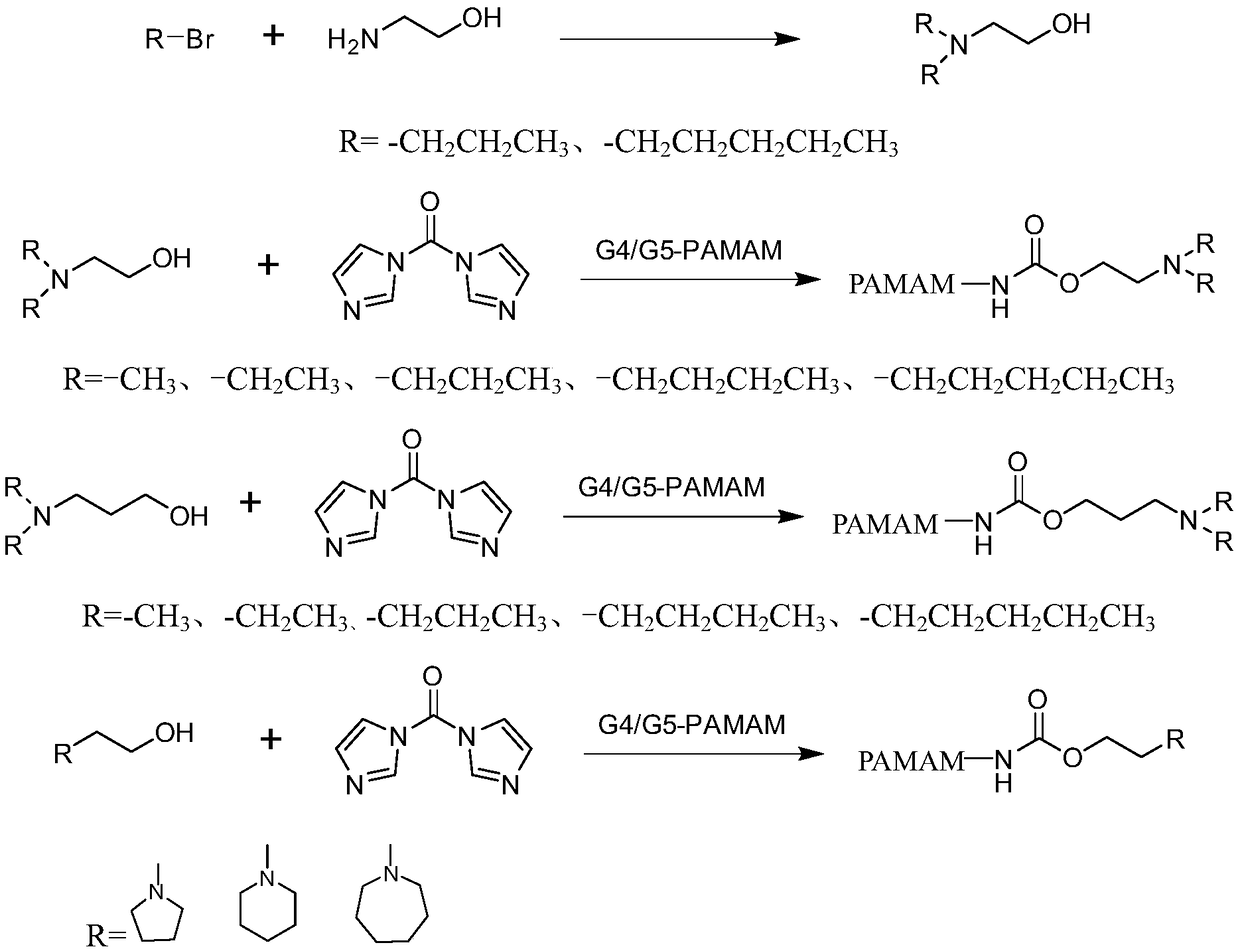
Modified dendritic PAMAM polymer as well as preparation method and application thereof
A polymer and dendritic technology, applied in the field of medical materials, can solve the problems of limited temperature adjustment range and single type of polymer, and achieve the effect of reducing toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: Dendrimer G4-PAMAM-DMEA 45 Synthesis
[0053] Dendrimer G4-PAMAM-DMEA 45 It is prepared by reacting the amino group on the surface of G4-PAMAM with the small molecule of tertiary amine activated by CDI (DMEA-CDI). The synthetic route is as follows: figure 1 shown.
[0054] Preparation of CDI-activated DMEA (DMEA-CDI):
[0055] Dissolve DMEA (N,N-dimethylethanolamine) (0.02mol) in 20mL of dichloromethane to obtain a DMEA solution; dissolve CDI (0.05mol) in 40mL of dichloromethane to obtain a CDI solution; drop the DMEA solution into CDI solution (complete dropwise addition within 30 minutes), after the dropwise addition is completed, react at room temperature for 24 hours; after the reaction is completed, wash the product three times with deionized water (collect the lower layer product), remove a small amount of water with anhydrous magnesium sulfate, and spin evaporate Remove the solvent dichloromethane, and then use a vacuum pump to remove a small amoun...
Embodiment 2
[0062] Example 2: Dendrimer G4-PAMAM-DEEA 45 Synthesis
[0063] Dendrimer G4-PAMAM-DEEA 45 It is prepared by reacting a small tertiary amine molecule (DEEA-CDI) activated by CDI with the amino group on the surface of PAMAM. The synthetic route is as follows figure 1 shown.
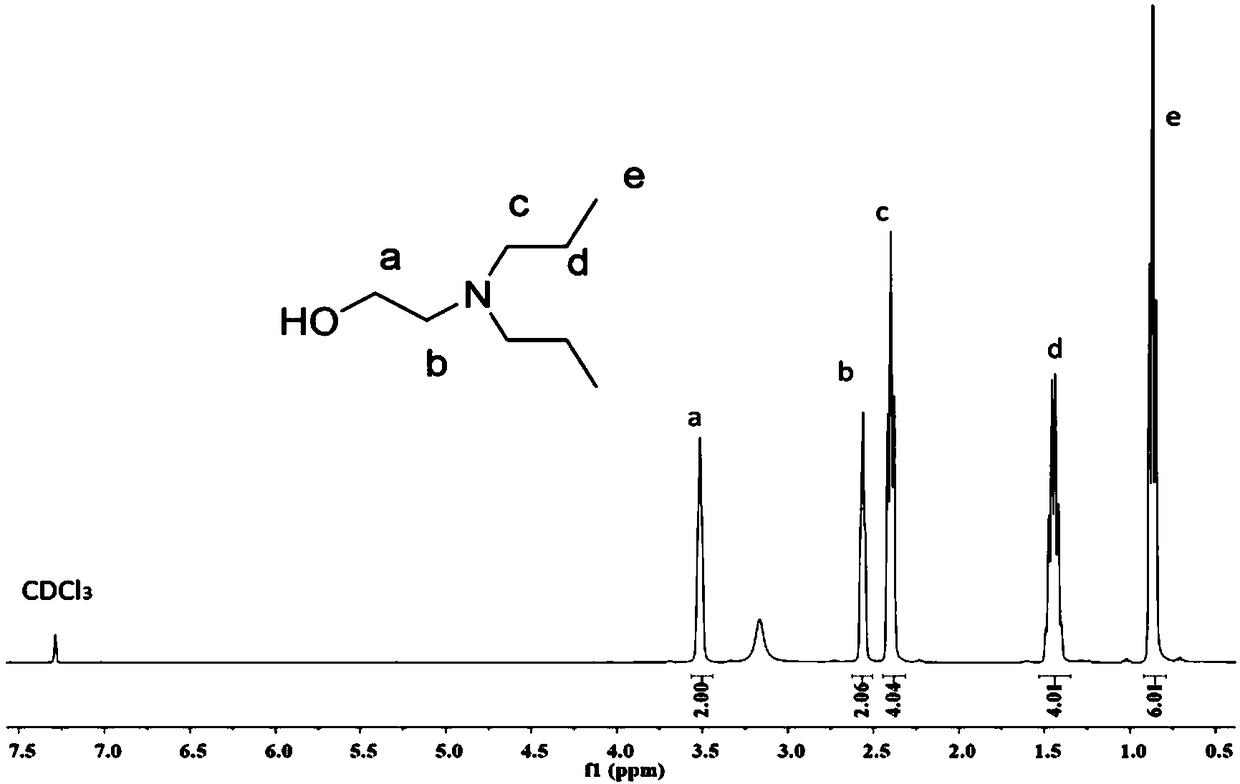
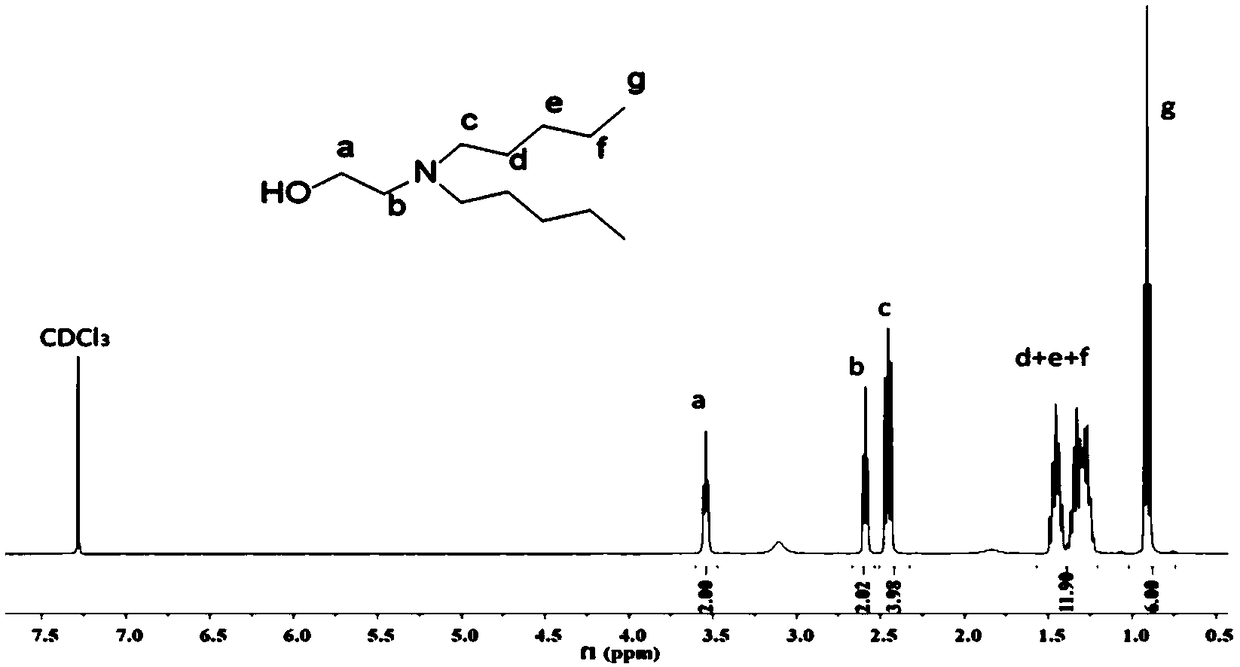
[0064] The preparation of DEEA activated by CDI, that is, DEEA-CDI: DEEA (N, N-diethylethanolamine) replaces DMEA (N, N-dimethylethanolamine) in Example 1, and other conditions and steps are the same as in Example 1, DEEA- The NMR results of CDI are shown in Fig. 3(b).
[0065] G4-PAMAM-DEEA 45 Preparation of:
[0066] (1) In 1.5ml of anhydrous DMSO, react 25mg of fourth-generation PAMAM (G4-PAMAM) with 71mg of DEEA-CDI in an oil bath at 40°C for 24 hours, the molar ratio of DEEA-CDI to the terminal amino group of G4-PAMAM The ratio is 3:1, and the product is obtained;
[0067] (2) Concentrate the product to about 0.5-1.0mL with a vacuum pump, pass through a Sephadex LH20 column, use methanol as the...
Embodiment 3
[0071] Example 3: Dendrimer G4-PAMAM-DPEA 45 Synthesis
[0072] Preparation of small molecules of N,N-dipropylethanolamine (DPEA) tertiary amine:
[0073] Dissolve ethanolamine (0.05 mol) and bromopropane (0.1 mol) in 100 mL of acetonitrile, add sodium carbonate (0.15 mol), and react under airtight conditions for 4 days at room temperature; filter the solution after the reaction to remove excess sodium carbonate and by-products Sodium bromide salt, the solvent was removed by rotary evaporation; the product was dissolved in dichloromethane, and then washed three times with saturated brine, the organic phase was collected, and the organic solvent was removed under reduced pressure. Colorless transparent liquid product with a yield of 44%. The NMR results of N,N-dipropylethanolamine (DPEA) are shown in Fig. 2(a).
[0074] The preparation of DPEA activated by CDI, that is, DPEA-CDI: DPEA (N, N-dipropylethanolamine) replaces DMEA (N, N-dimethylethanolamine) in Example 1, and oth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cloud point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com