Frog-derived wound repair peptide and synthesis method thereof
A wound repair and synthesis method technology, applied in the field of biomedicine, can solve the problems of reduced yield of target polypeptide, low renaturation efficiency, hindered healing, etc., and achieves the effect of simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
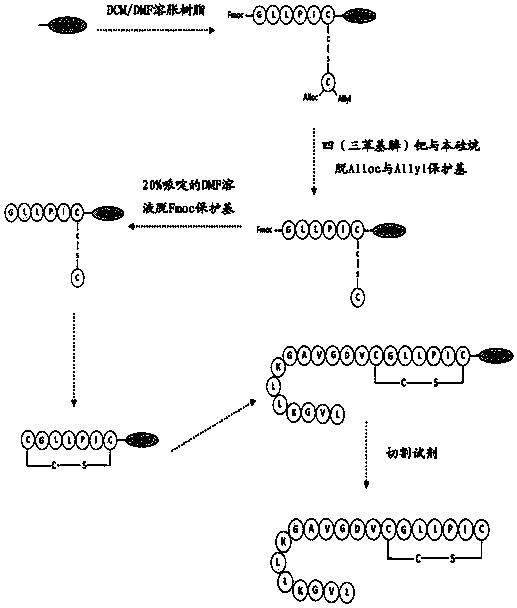
[0061] The present invention also provides a method for synthesizing the frog wound repair peptide shown in SEQ ID No.1, characterized in that the method mainly includes the following steps:
[0062] The Fmoc solid-phase synthesis method is adopted, and the resin is used as a solid-phase carrier, which is activated by a condensing agent. The first amino acid (position 1, counted from the C-terminal to the N-terminal) connected to the solid-phase resin is a diaminodiacid, and the amino acids are coupled one by one from the C-terminal to the N-terminal according to the sequence shown in SEQ ID No.1. on the resin. After the amino acid in the sequence shown in SEQ ID No.1 is condensed from the C-terminal to the 6th glycine at the N-terminal, the protecting group of the diaminodiacid is removed using a removing reagent. Afterwards, the diaminodiacid at the first position from the C-terminal to the N-terminal is cyclized and condensed with the amino acid at the sixth position. Nex...
Embodiment 1
[0084] Example 1: Conventional preparation method of frog wound repair peptide as shown in SEQ ID No.1
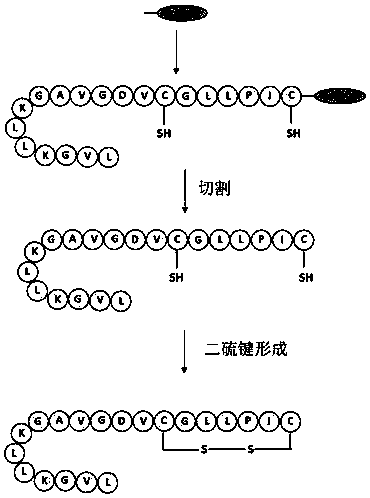
[0085] The amino acid sequence of one of the frog wound repair peptides provided in this application is shown in SEQ ID No. 1, which is LVGKL LKGAV GDVCG LLPIC. In the routine synthesis of frog wound repair peptides, linear peptides need to be synthesized first, and a denaturation process is required after cleavage.
[0086] The flowchart of the traditional method for synthesizing frog wound repair peptide as shown in SEQ ID No.1 is as follows figure 1 shown in .
[0087] The specific steps of synthesis are as follows:
[0088] Weigh 2-Cl-Trt resin or wang resin (0.125mmol) into a solid-phase synthesis tube, and use DCM / DMF to swell the resin for 15-30min. Drain the solvent, wash with DCM, and carry out condensation reactions in sequence according to the polypeptide sequence. Polypeptide chain synthesis (Fmoc-amino acid 0.4mmol, HCTU 0.38mmol, DIEA 8mmol) was carried ou...
Embodiment 2
[0093] Example 2: The frog wound repair peptide shown in SEQ ID No.1 was synthesized by the method of the present application
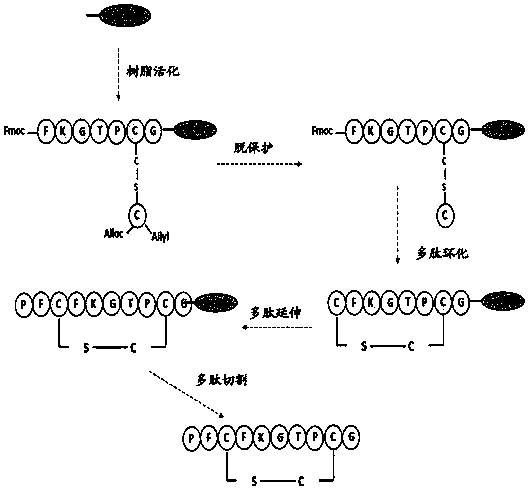
[0094] The amino acid sequence of the frog wound repair peptide provided by this application is shown in SEQ ID No.1, which is LVGKL LKGAV GDVCG LLPIC, and wherein the S atom on the first Cys from the C-terminal to the N-terminal is replaced by a C atom Replaced, thus causing the S-S bond between the 1st and 7th positions from the C-terminal to the N-terminal to be replaced with a C-S bond.
[0095] The flow chart of the synthesis of frog wound repair peptide shown in SEQ ID No.1 is as follows Figure 4 shown in .
[0096] The specific steps of synthesis are as follows:
[0097] Weigh 2-Cl-Trt resin (0.125 mmol) into a solid-phase synthesis tube, and use DCM / DMF to swell the resin for 15-30 min. Drain the solvent, wash with DCM, and carry out condensation reactions in sequence according to the polypeptide sequence.
[0098] The first amino acid (p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com