A kind of benzothiazole-based mitochondrial pH fluorescent probe and preparation method thereof
A technology of methylbenzothiazole and fluorescent probe, which is applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of long-distance difference and low sensitivity, and achieve reduced interference, high sensitivity, and excellent mitochondrial target The effect of orientation ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1, compound 3-(6-bromohexyl)-2-methylbenzothiazole-3-bromide (BMBI), compound 2-methyl-3-(6-(triphenylphosphoryl)hexyl)benzo Thiazole-3-bromide (MTBI) and the probe 2-(2-(6-hydroxynaphthalen-2-yl)vinyl)-3-(6-(triphenylphosphono)hexyl)benzothiazole-3 - Preparation of Bromide (HTBT2):
[0031]
[0032] (1) A mixed solution of 2-methylbenzothiazole (15 mmol, 1.90 mL) and 1,6-dibromohexane (75 mmol, 12.11 mL) was reacted in a locked tube at 140° C. for 5 h. After the reaction was cooled to room temperature in CH 2 Cl 2 The product (5.43 g, 92%) was obtained as a gray solid by precipitation with diethyl ether. 1 H NMR (400MHz, CDCl 3 )δ8.32(d, J=8.1Hz, 1H), 8.06(d, J=8.4Hz, 1H), 7.81(t, J=7.7Hz, 1H), 7.69(t, J=7.7Hz, 1H) ,5.09–4.75(m,2H),3.49(s,3H),3.40(t,J=6.5Hz,2H),2.25–1.75(m,8H). 13 C NMR (101MHz, CDCl 3 )δ175.69,140.93,130.00,129.23,128.67,124.72,116.57,51.00,33.77,32.28,28.67,27.68,25.98,19.30.MS(ESI-MS):m / z Calcd 313.0402[M] + ;found 312.0416,314.0387[M] +...
Embodiment 2
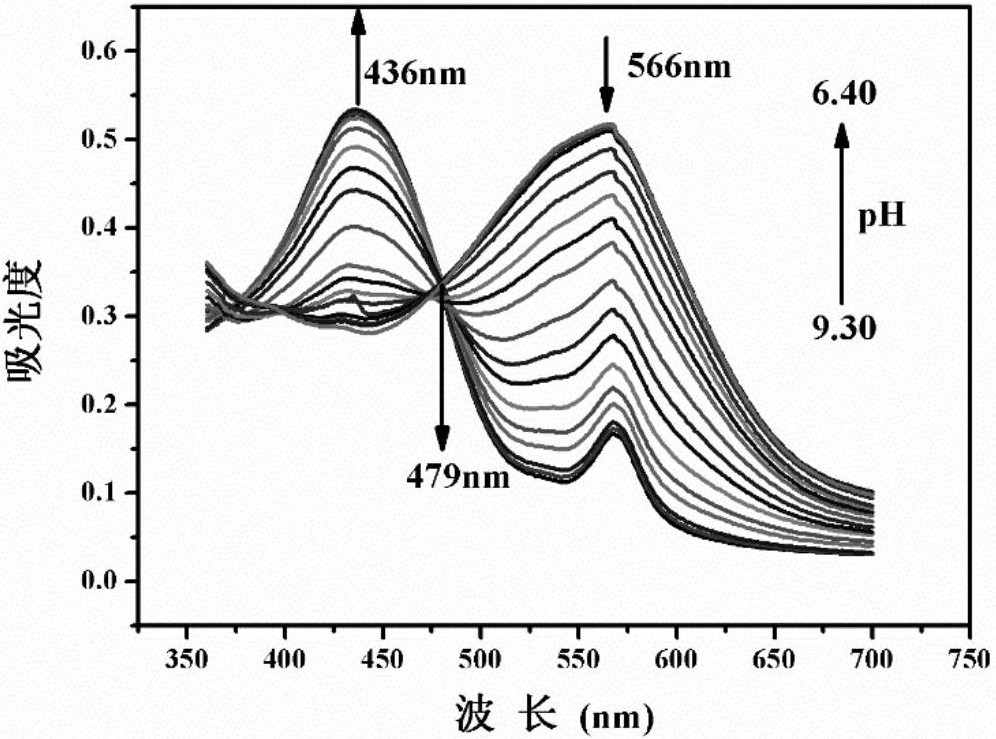
[0036] The concentration of the probe HTBT2 in Example 1 was maintained at 200 μM, and Tris-hydrochloric acid buffer solution (V DMSO :V H2O =2:1,0.05M) measured its absorption spectrum in the system ( figure 1 ). As the pH value decreases from 9.30 to 6.40, the absorption peak at 566nm decreases gradually, the absorption peak at 436nm increases correspondingly, and there is an isosbestic point at 479nm. The color of the solution also changed from the original yellow to yellow-green ( figure 2 ).
Embodiment 3
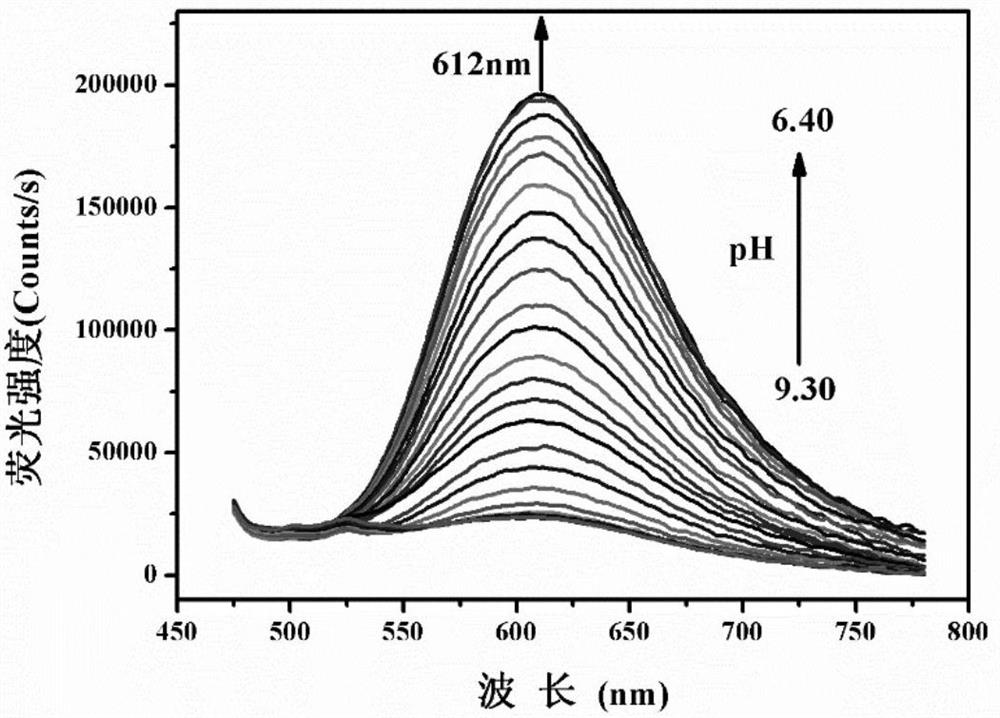
[0038] The probe concentration in Example 1 was kept at 10 μM, and its fluorescence emission spectrum was measured in Tris-hydrochloric acid buffer (VDMSO:VH2O=2:1, 0.05M) system with different pHs, and the fixed excitation wavelength was 436nm ( image 3 ). As the pH value decreased from 9.30 to 6.40, the solution had a new fluorescence emission peak at 612nm and gradually increased. The color of the solution changed from colorless to orange-red ( Figure 4 ). By fitting the fluorescence intensity value of HTBT2 at 612nm with the Boltzmann function of pH value, the calculated pKa value was 8.04±0.02( Figure 5 ), the pH response linear range is 7.20-8.70. The linear regression equation is F=764314.81192-79080.76184×pH, the correlation coefficient R 2 =0.9991( Figure 6 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com