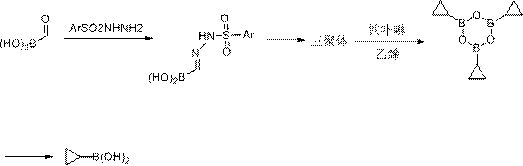
A kind of synthetic method of cyclopropyl boronic acid
A technology of cyclopropylboronic acid and aldehydeboronic acid, which is applied in the synthesis of alkylboronic acid and cyclopropylboronic acid, and can solve the problems of large amount of alkali and easy flushing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] In the reaction flask, add aldehyde boronic acid (7.75g, 0.105mol), absolute ethanol 65mL and 2,4-dinitro-p-toluenesulfonyl hydrazide (26.2g, 0.1mol), heat up to reflux reaction after adding, TLC After the detection reaction was completed, the absolute ethanol was distilled off under normal pressure, and then 220 mL of toluene was added and then refluxed to separate water until the detection reaction was completely transformed into a trimer. After cooling down, evaporate the toluene solvent to dryness, cool down to 0°C, add 160mL of 1,2-dichloroethane and catalyst FeCl(TPP)(0.15eq) (note: the equivalent is aldehyde boronic acid 0.15eq, the same below) and start to Continuously feed ethylene gas into the reaction solvent, replace the gas outlet of the reaction bottle with two layers of balloons, maintain a certain pressure in the reaction system until the system no longer absorbs, add 60mL sulfolane, and distill under reduced pressure to obtain cyclopropyl boric acid trim...
Embodiment 2
[0025] In the reaction flask, add aldehyde boronic acid (7.4g, 0.1mol), absolute ethanol 65mL and 2,4-dinitro-p-toluenesulfonyl hydrazide (26.2g, 0.1mol), after adding, heat up to reflux reaction, TLC After the detection reaction was completed, the absolute ethanol was distilled off under normal pressure, and then 220 mL of toluene was added and then refluxed to separate water until the detection reaction was completely transformed into a trimer. After cooling down, evaporate the toluene solvent to dryness, cool down to 0°C, add 120mL of tetrahydrofuran, then add 1.6M n-butyllithium (65mL, 0.104mol) dropwise, stir for 30 minutes, add catalyst FeCl(TPP) (0.05eq) , began to continuously feed ethylene gas into the reaction solvent, replaced the gas outlet of the reaction bottle with two layers of balloons, maintained a certain pressure in the reaction system until the system no longer absorbed, added 60mL sulfolane, and distilled under reduced pressure to obtain cyclopropyl Add 3...
Embodiment 3
[0027] In the reaction flask, add aldehydoboronic acid (7.4g, 0.1mol), anhydrous methanol 60mL and 3,5-dinitro-p-toluenesulfonyl hydrazide (26.2g, 0.1mol), heat up to reflux reaction after addition, TLC After the detection reaction was completed, the anhydrous methanol was distilled off under normal pressure, and then 220 mL of toluene was added, and then the water was refluxed until the detection reaction was completely transformed into a trimer. After cooling down, evaporate the toluene solvent to dryness, cool down to 0°C, add 120mL of tetrahydrofuran, then add 2.5M n-butyllithium (65mL, 0.104mol) dropwise, stir for 30 minutes, add the catalyst FeBr(TPP) (0.05eq) , began to continuously feed ethylene gas into the reaction solvent, replaced the gas outlet of the reaction bottle with two layers of balloons, maintained a certain pressure in the reaction system until the system no longer absorbed, added 60mL sulfolane, and distilled under reduced pressure to obtain cyclopropyl ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com