Functional quinoline liquid corrosion inhibitor, preparation method and application
A quinoline and corrosion inhibitor technology, applied in the field of preparation and functionalized quinoline liquid corrosion inhibitor, achieves the effects of simple operation, simple processing and low vapor pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
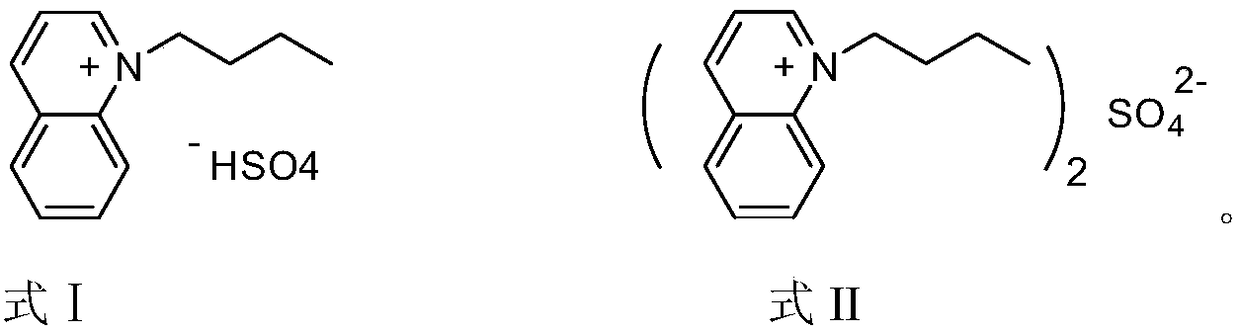
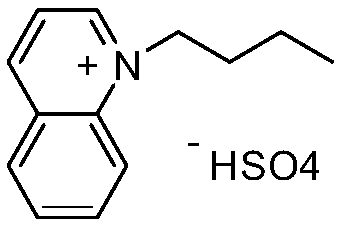
[0022] The structural formula of 1-butylquinoline hydrogen sulfate ionic liquid is:
[0023]
[0024] Preparation Process:
[0025] Add 0.1 mol of quinoline to a 100 mL three-necked flask, add 0.1 mol of n-butane bromide to the three-necked flask at room temperature, stir for 10 minutes after the addition is complete, and react under reflux for 22 hours. The reaction solution is cooled and washed with 10 mL of ether to obtain a sticky solution. Thick substance, namely quinoline ionic liquid functionalized with n-butyl group. Then add 20 mL of dichloromethane to the three-necked flask, maintain the temperature of the system below 5°C, add 0.06 mol of concentrated sulfuric acid dropwise, and after the dropwise addition is completed, heat and stir to reflux for 21 hours. The dichloromethane was evaporated at elevated temperature, and the product was washed with 10 mL of ether after cooling. After the ether was separated and removed, 1-butylquinoline hydrogen sulfate ionic liquid (for...
Embodiment 2
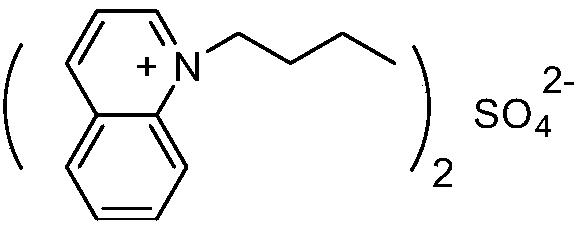
[0027] The structural formula of 1-butylquinoline sulfate ionic liquid is:
[0028]
[0029] Add 0.1 mol of quinoline to a 100 mL three-necked flask, add 0.1 mol of n-butane bromide to the three-necked flask at room temperature, stir for 10 minutes after the addition is complete, and react under reflux for 22 hours. The reaction solution is cooled and washed with 10 mL of ether to obtain a sticky solution. Thick substance, namely quinoline ionic liquid functionalized with n-butyl group. After that, 20 mL of dichloromethane was added to the three-necked flask to maintain the temperature of the system below 5° C., 0.03 mol of concentrated sulfuric acid was added dropwise, and after the addition was completed, the mixture was heated, stirred and refluxed for 21 hours. The dichloromethane was evaporated at elevated temperature, and the product was washed with 10 mL of ether after cooling. After separating and removing the ether, the 1-butylquinoline sulfate ionic liquid (formula II) ...
Embodiment 3
[0031] The most intuitive and easiest way to evaluate corrosion inhibition performance is to compare the weight of steel sheets before and after corrosion by the weightless method (static coupon test). Then through a series of data inference to get the final corrosion inhibition efficiency. Therefore, we took 1-butylquinoline hydrogen sulfate ionic liquid as an example to study its corrosion inhibition performance on low carbon steel in 1 mol / L hydrochloric acid solution.
[0032] We first evaluated the inhibitory rate of (formula I) ionic liquids of different concentrations in 1 mol / L hydrochloric acid solution at 30°C on the corrosion of steel sheets by hydrochloric acid. The experimental data is shown in Table 1-1:
[0033] Table 1-1 (Formula I) Corrosion Inhibition Performance of Ionic Liquid in 1mol / L Hydrochloric Acid at 30℃
[0034]
[0035] From the data in Table 1-1, we can see that, compared with blank hydrochloric acid, the addition of 1-butylquinoline hydrogen sulfate i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com