Preparation method of nitrofurazone alpha crystal
A technology for nitrofurazone and crude nitrofurazone, which is applied in the field of drug crystallization, can solve the problems of non-concentrated particle size distribution, potential safety hazards, and small bulk density, and achieve the effects of increasing bulk density, good stability, and improving filtration properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Add 15 g of dry nitrofurazone β crystal form to a mixed solution of 50 g N,N-dimethylformamide and 50 g methanol to form a suspension, keep the temperature of the suspension at 5°C while stirring, suspend and stir for 24 hours, and filter the suspension with suction , the product was dried to constant weight at 50°C under normal pressure to obtain nitrofurazone in α crystal form.
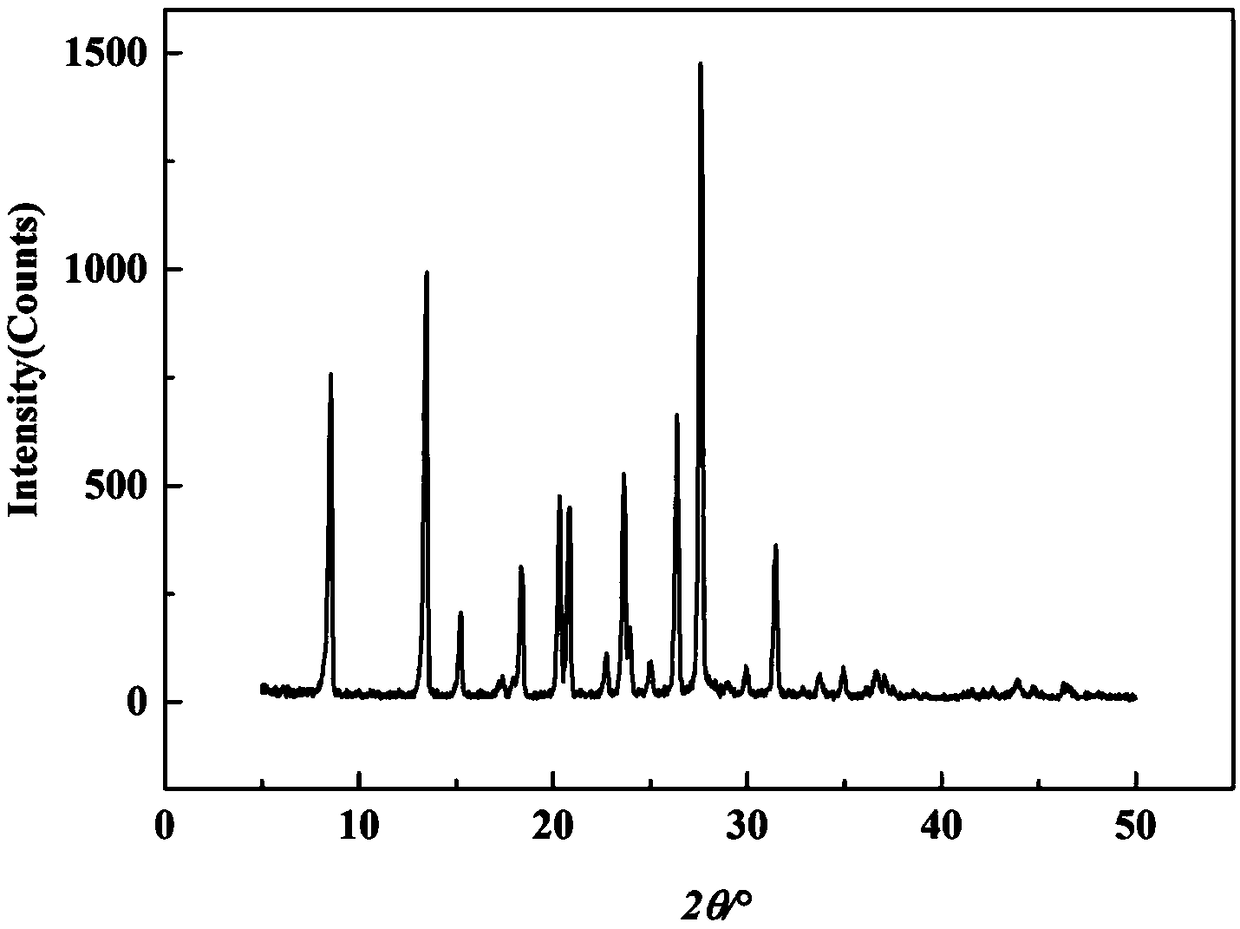
[0036] The X-ray powder diffraction of the crystal obtained in this embodiment is as follows: figure 1 As shown, it mainly has characteristic peaks at diffraction angles 2θ=8.782, 13.719, 15.443, 18.660, 20.581, 21.081, 22.998, 23.899, 24.200, 25.317, 26.638, 27.877, 30.238, 31.700, 35.182 °. The more comprehensive characteristic peaks are 衍射角2θ=8.782,13.719,15.443,17.622,18.660,20.581,21.081,22.998,23.899,24.200,25.317,26.638,27.877,30.238,31.700,33.202,34.001,35.182,36.880,37.321°,所以所得产品为α Crystalline nitrofurazone,
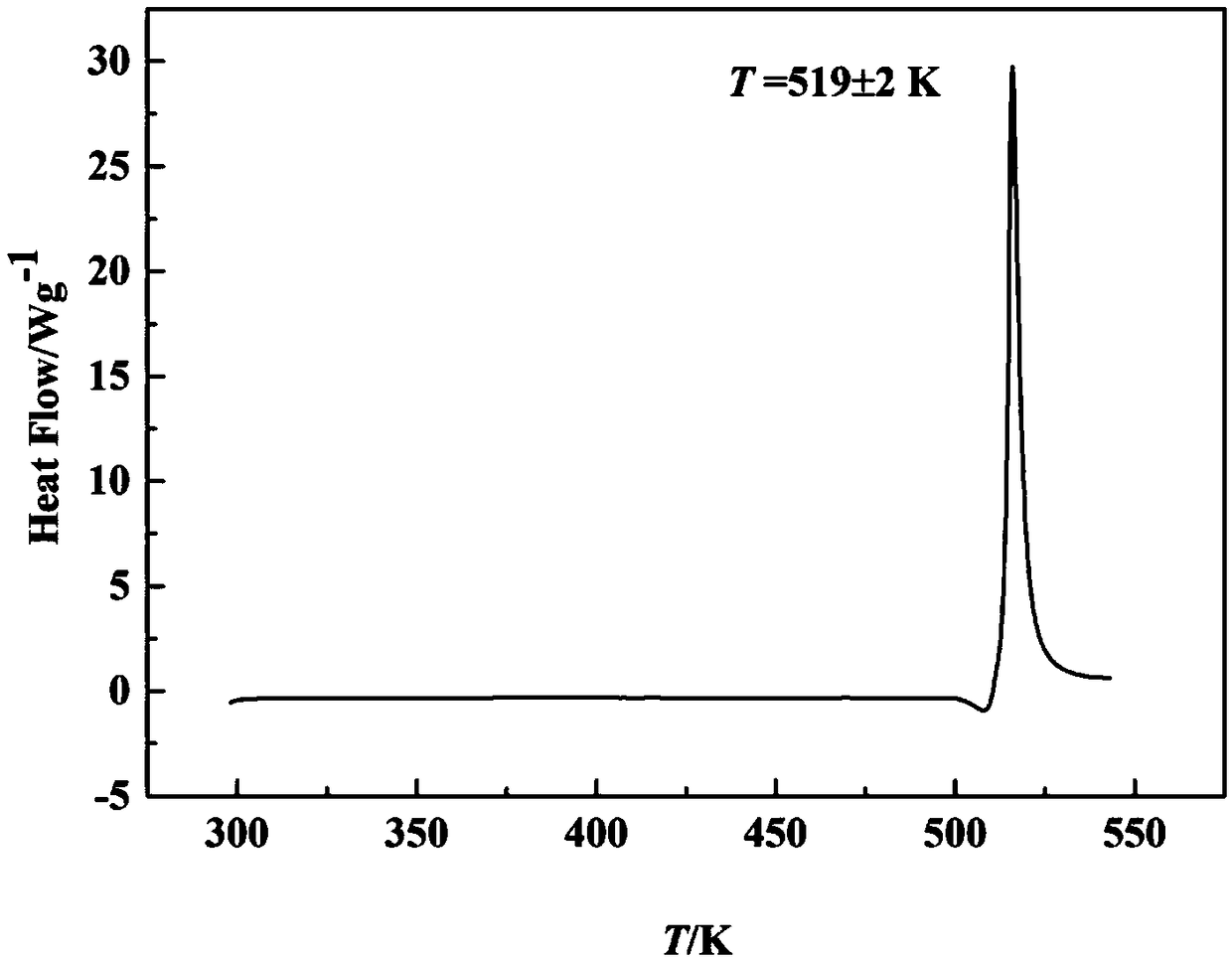
[0037] The characteristic peak temperature of the DSC diagram of the nit...
Embodiment 2
[0042] Add 16 g of dry nitrofurazone β crystal form to a mixed solution of 50 g formamide and 50 g acetonitrile to form a suspension, keep the temperature of the suspension at 20°C under stirring, suspend and stir for 11 hours, filter the suspension with suction, and put the product at 50°C , and dry to constant weight under normal pressure to obtain α-crystalline nitrofurazone.
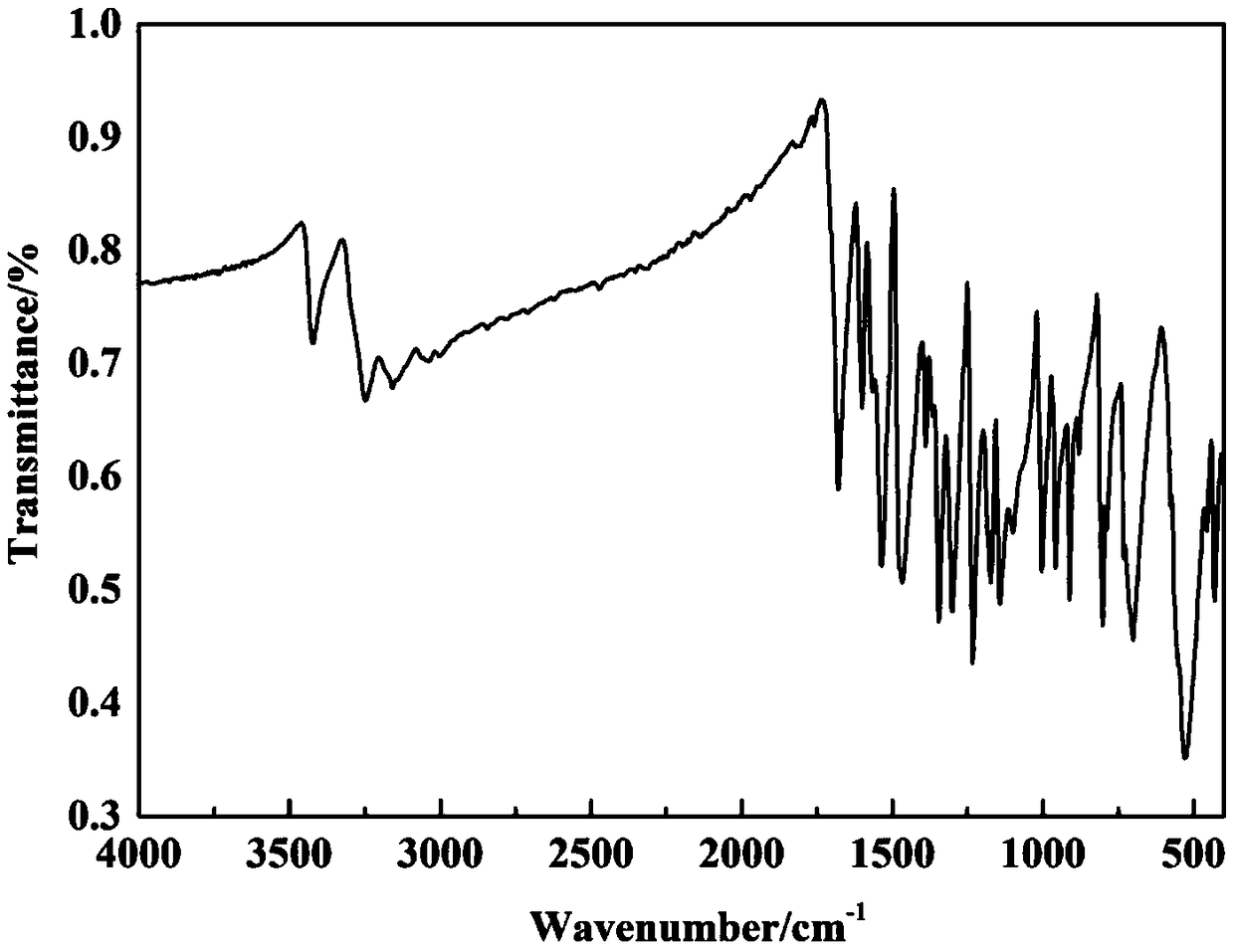
[0043] The X-ray powder diffraction, DSC, infrared and Raman spectrum characterization data of the crystal obtained in this example are the same as those in Example 1, the UV purity is 98.62%, and the bulk density is 0.36g / cm 3 .
Embodiment 3
[0045] Add 2.8 g of dry nitrofurazone β crystal form to 10 g of dimethyl sulfoxide to form a suspension, keep the temperature of the suspension at 25°C under stirring, suspend and stir for 15 hours, filter the suspension with suction, and store the product at 80°C under normal pressure Drying to constant weight at 100°C yields α crystalline nitrofurazone.
[0046] The X-ray powder diffraction, DSC, infrared and Raman spectrum characterization data of the crystal obtained in this example are the same as those in Example 1, the UV purity is 98.56%, and the bulk density is 0.36g / cm 3 .
[0047] Ultrasound-assisted solvent-mediated crystallization:
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com