Use of Talazoparib in preparation of drugs for treating or preventing diseases related with hepatitis virus
A technology of hepatitis virus and hepatitis B virus, which is applied in the field of medicine, can solve the problems of enhanced radiation toxicity effect, inapplicability, enhanced cytotoxicity of radiotherapy and chemotherapy, etc., and achieve the effect of improving the therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
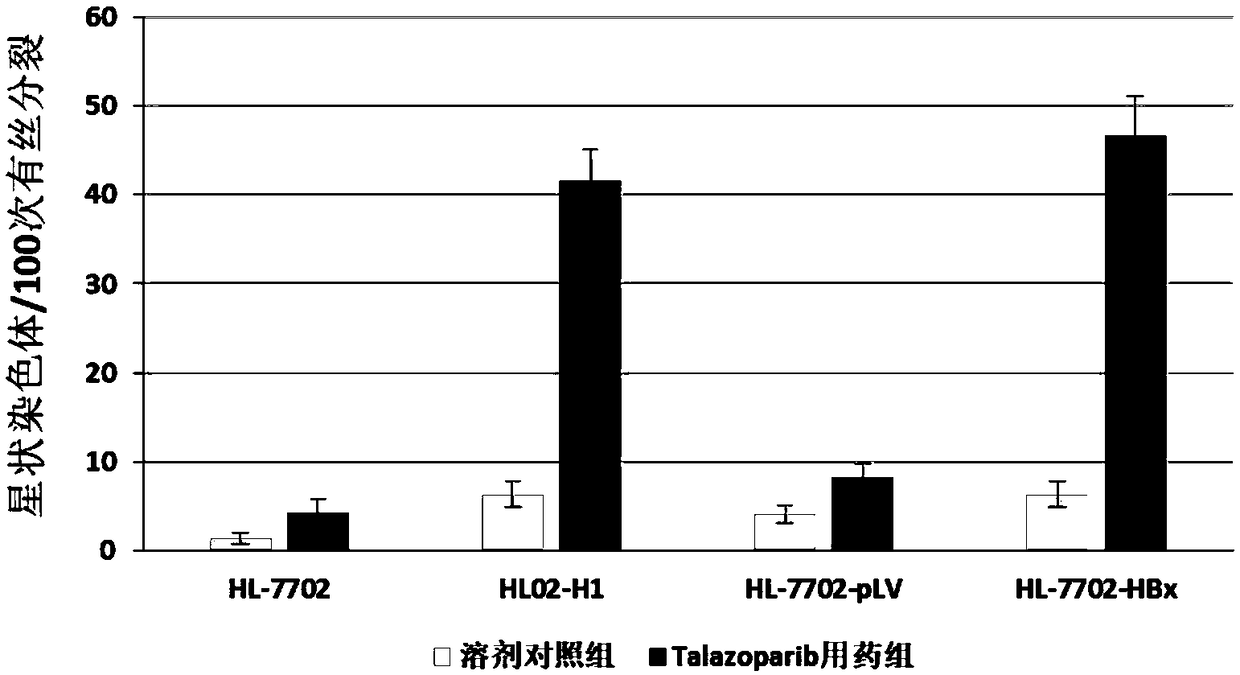
[0057] Example 1 Taraparib induces the expression of HBx and the chromosomal aberration of the liver cells carrying hepatitis B virus. experimental method:
[0058] 1. HBx protein expression and construction of HBV genome integrated cell line.
[0059]HL02-H1 is a human liver cell line that stably expresses the entire HBV genome. HL02-H1 was deposited in China Center for Type Culture Collection (CCTCC, accession number: C201554). The HBx protein expression cell HL-7702 / HBx and its control cell HL-7702 / pLV were constructed by a lentivirus infection system. The specific methods of lentivirus packaging and infection are conventional techniques and will not be repeated here.
[0060] 2. Taraparib induces chromosomal aberrations in HBx protein expressing cells and hepatitis B virus host cells.
[0061] HL-7702, HL02-H1, HL-7702 / pLV and HL-7702 / HBx cells were respectively inoculated into two 6cm diameter culture dishes, and after overnight culture, each cell was added with tarapa...
Embodiment 2
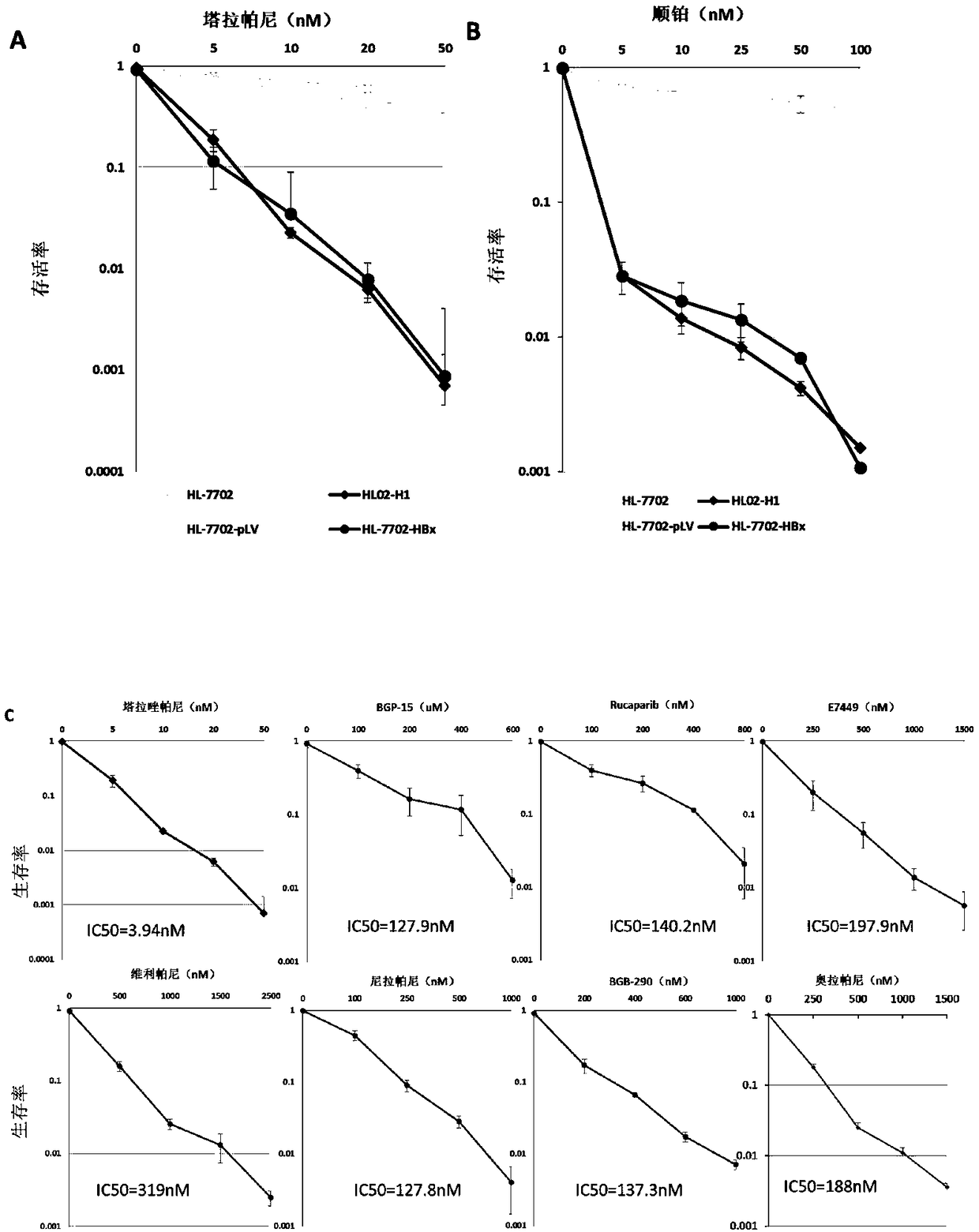
[0066] Example 2 Low-dose tarapanib specifically inhibits the expression of HBx and the proliferation of hepatitis B virus host liver cells
[0067] experimental method:
[0068] Colony formation experiments were used to further verify the specific inhibitory effect of taraparib on hepatocytes expressing HBx and hepatitis B virus host.
[0069] HL-7702, HL02-H1, HL-7702 / pLV and HL-7702 / HBx cells were seeded in 10 cm diameter culture dishes respectively, and 800 cells were added to each culture dish. After culturing overnight, add tarapanib or the same volume of DMSO (control group) the next day. After 10 days of continuous treatment with tarapanib, the cells were fixed with methanol, stained with Giemsa stain, and the number of clones was counted. All experiments were repeated 3 times.
[0070] Drug management includes taraparib monotherapy and its combination with platinum compounds (cisplatin). The concentration of taraparib single drug treatment is 0, 5, 10, 20, 50nM; t...
Embodiment 3
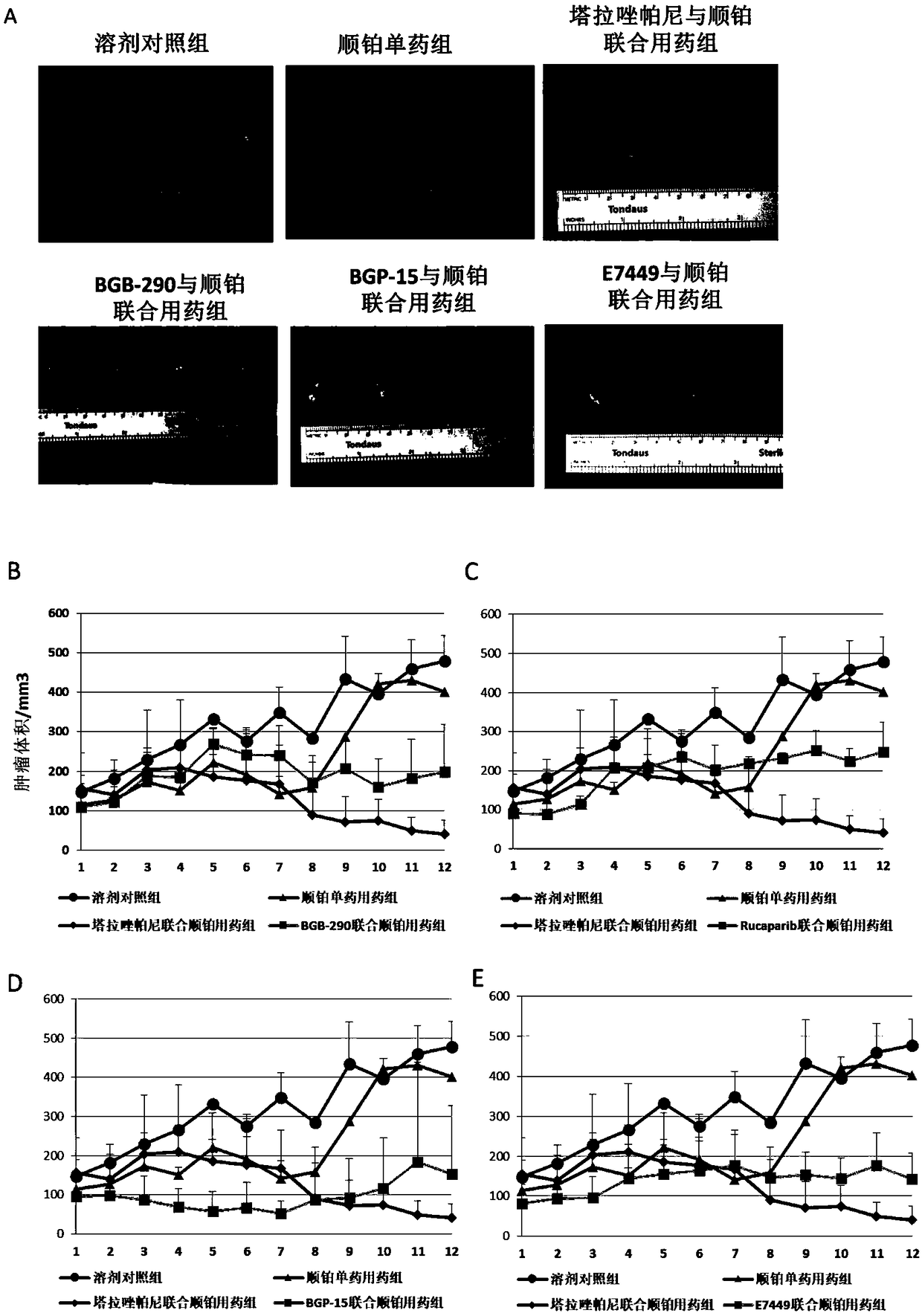
[0081] The curative effect of embodiment 3 taraparib to hepatitis B virus positive liver cancer
[0082] experimental method
[0083] Twenty-seven 6-week-old athymic female nude mice were randomly divided into 9 groups, each mouse was inoculated with HL02-H1 in the armpit and groin, and the inoculation quantity was 4x10 6 . The tumor volume is about 70-100mm 3 start injecting the drug. The drug injection regimen was a combination of taraparib and platinum drugs (cisplatin), and a solvent control group (DMSO) and a cisplatin single drug control group were set up. The specific dosages are as follows: the combined dosage of taraparib is 2.5mg / kg / day, and the dosages of other inhibitors are: BGB-290: 10mg / kg / day, BGP-15: 10mg / kg / day, E7449: 4mg / kg / day, Lukaparib: 10mg / kg / day, cisplatin dosage 3mg / kg, twice a week. The drug injection time was 12 days, and the tumor surface volume was measured on days 2, 5, 7, 8, 9, and 12. Tumor volume was calculated according to the followin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com