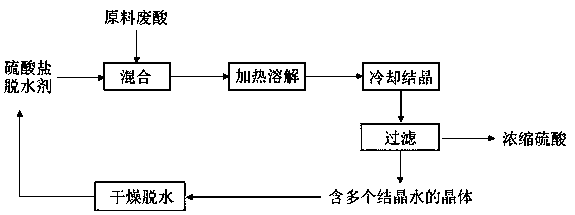
Process for concentrating titanium white waste acid through generation of crystalline hydrate by utilizing phase equilibrium principle
A technology for crystallization of hydrate and titanium white waste acid, which is applied in the field of chemical engineering, can solve the problems of limited concentration and long time consumption, and achieve the effects of improving crystallization efficiency, less equipment investment, and improving crystallization process and phase balance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Get 100g of dilute sulfuric acid solution containing saturated ferrous sulfate respectively (acid concentration is 15.98wt%, FeSO 4 The content is 15.77wt%. ), 34g of anhydrous ferrous sulfate in a 250ml three-necked flask, placed the three-necked flask in a constant temperature water bath, kept the temperature at 40°C for 2h to promote dissolution and hydrate formation, then crystallized at 25°C for 8h, and separated by filtration. The results showed that the concentration of sulfuric acid after separation increased to 29.45wt%. The isolated solid was identified as ferrous sulfate heptahydrate.
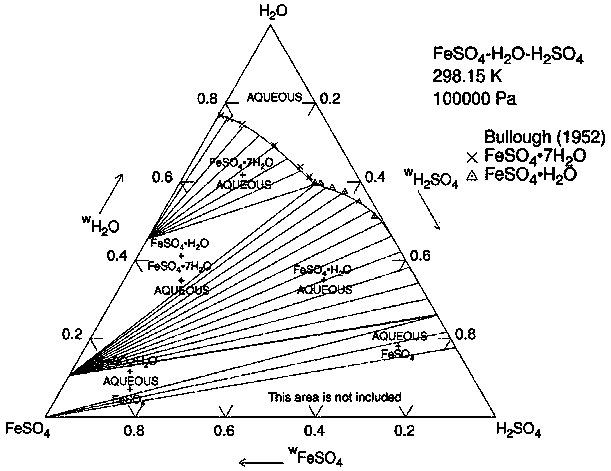
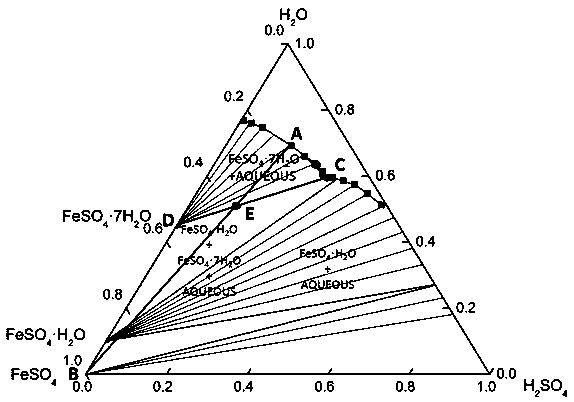
[0022] The principle of this embodiment is shown in image 3 The ternary phase diagram of the sulfuric acid-ferrous sulfate-water system at 25°C is shown. The state of the initial dilute acid is point A in the figure, and it is connected with the vertex B of anhydrous ferrous sulfate to form a straight line. To obtain the concentrated sulfuric acid composed of point C by c...
Embodiment 2
[0024] Take respectively 50g of dilute sulfuric acid solution containing saturated ferrous sulfate (acid concentration is 15.98wt%, FeSO 4The content is 15.77wt%. ), 220g of anhydrous ferrous sulfate in a 250ml three-neck flask, place the three-necked flask in a constant temperature water bath, keep the temperature at 40°C for 2h to promote dissolution and hydrate formation, then crystallize at 25°C for 8h, and separate by filtration. The results showed that the concentration of sulfuric acid after separation increased to 64.02wt%. The isolated solid was identified as ferrous sulfate monohydrate.
[0025] The principle of this embodiment is shown in Figure 4 The ternary phase diagram of the sulfuric acid-ferrous sulfate-water system at 25°C is shown. The state of the initial dilute acid is point A in the figure, and it is connected with the vertex B of anhydrous ferrous sulfate to form a straight line. To obtain the concentrated sulfuric acid composed of point C by crysta...
Embodiment 3
[0027] Aluminum sulfate octadecahydrate is dried to produce anhydrous aluminum sulfate. Take 100g of raw material dilute acid (acid concentration is 20.00wt%), put 70g of anhydrous aluminum sulfate in a 250ml three-necked flask, place the three-necked flask in a constant temperature water bath, raise the temperature at 40°C for 2h to promote dissolution and hydrate formation, 25 It was crystallized at ℃ for 7h and separated by filtration. The concentration of spent acid was increased to 56.10%. The separated solid is identified as aluminum sulfate hexadecahydrate, and it can be reused after drying to remove crystal water.
[0028] The principle of this embodiment is shown in Figure 5 The ternary phase diagram of the sulfuric acid-aluminum sulfate-water system at 25°C is shown. The state of the initial dilute acid is point A in the figure. Connect it with the vertex B of anhydrous aluminum sulfate to form a straight line. To obtain concentrated sulfuric acid composed of p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com