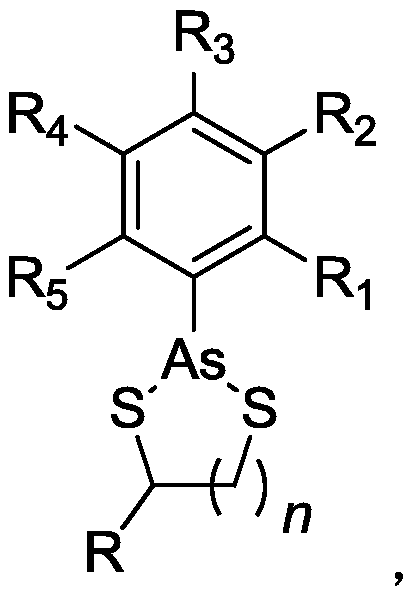
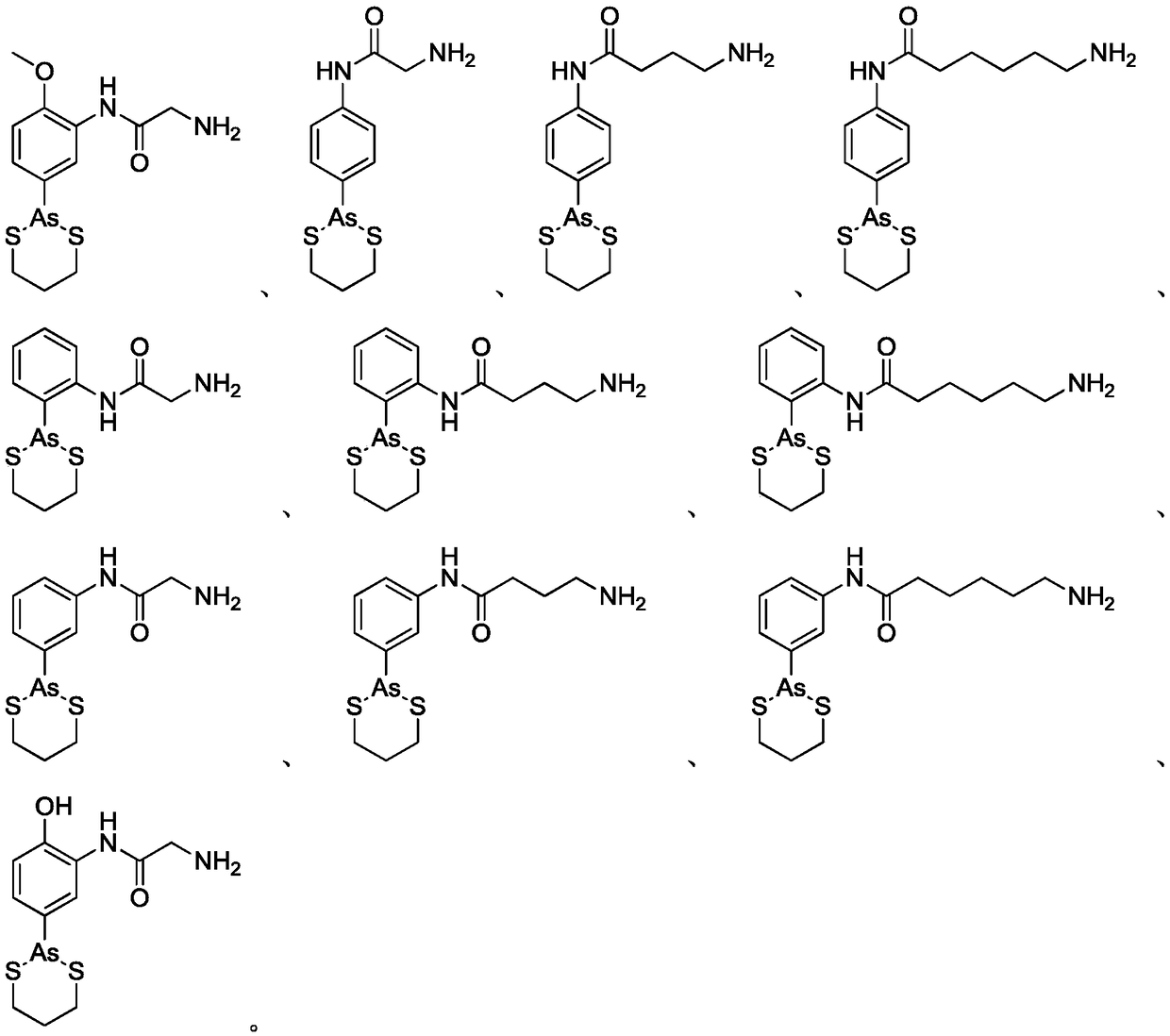
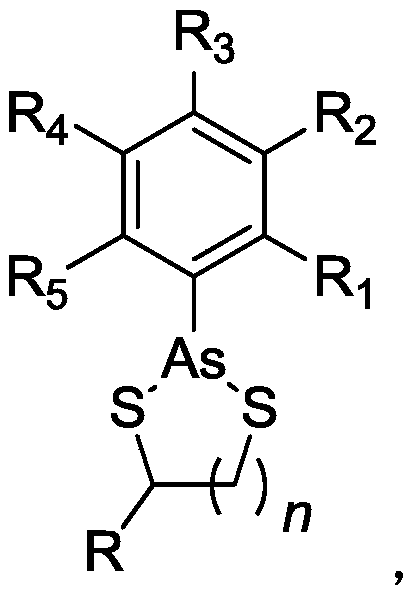
Organoarsenic compound and use thereof
A compound, organic arsenic technology, applied in the field of medicine, can solve problems such as drug obstruction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] ①Preparation of m-nitrophenylfluoroborate diazonium salt: firstly, 31.5mL of concentrated hydrochloric acid and 25mL of water were mixed uniformly, and then 17.25g of m-nitroaniline was added into it, and stirred uniformly. Placed in a low temperature reactor, 0 degrees Celsius. When the cooling is complete, the sodium nitrite solution (8.65 g of sodium nitrite dissolved in 20 mL of water) is dropped into the above ice bath solution, and the end point is indicated by starch potassium iodide. After the diazonium salt is prepared, the sodium fluoroborate solution (27.5 g of sodium fluoroborate dissolved in 55 mL of water) is still dropped into the diazonium salt solution in a low temperature reactor at 0 degrees Celsius. It can be observed that the reaction solution is getting more and more turbid and thick. After 30 minutes of reaction, the reaction is lifted, suction filtration is carried out, and the liquid is no longer left after suction filtration, and the filter cak...
Embodiment 1
[0064] Target:
[0065] Mix 30 mL of methanol and 24 mL of concentrated hydrochloric acid, then add 13.2 g of roxarsone into it, and finally add 100 mg of potassium iodide. During the stirring process, sulfur dioxide gas is introduced, and the reaction is terminated for about half an hour. Stir under ice bath conditions, and slowly add concentrated ammonia water. , until the pH is approximately equal to 8, the reaction solution is allowed to stand, the reaction solution is layered, the upper layer liquid is poured out, a viscous yellow solid is obtained in the lower layer, and ethanol is added to dissolve the viscous substance. During the dropwise addition, a large amount of bright yellow solid precipitates were generated. After the dropwise addition, the reaction was refluxed for half an hour, cooled, and the solid product was filtered. The filter cake was rinsed with ethanol, and the filtrate was combined for column chromatography to obtain 5.7 g of product. This product w...
Embodiment 2
[0070] Target:
[0071] Mix 30 mL of methanol and 24 mL of concentrated hydrochloric acid, then add 10.9 g of p-aminophenylarsenic acid to it, and finally add 100 mg of potassium iodide. During the stirring process, sulfur dioxide gas is introduced, and the reaction is terminated for about half an hour. At this time, the solution is in a turbid state. Suction filtration, add the filter residue to the flask, add concentrated ammonia water to adjust the pH to about 8, and suction again to obtain an off-white solid product, which is added to a round-bottomed flask, and then an appropriate amount of ethanol and ethanedithiol are added, and refluxed for half an hour , a white final product was obtained after column chromatography.
[0072] The reaction is as follows:
[0073]
[0074] Yield: 47%; 1 H NMR (CDCl 3 , 400MHz) δ: 7.441-7.408 (d, 2H, J=8.4Hz, Ar-H), 6.679-6.646 (d, 2H, J=8.4Hz, Ar-H), 3.792 (s, 2H, NH 2 -H), 3.380-3.310 (m, 2H, -S- CH 2 -CH 2 -S-), 3.252-3.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com