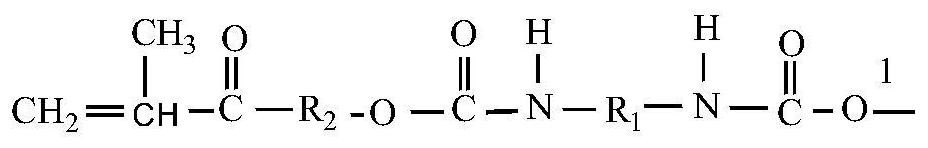Synthesis of a Polymer Acrylate Resin with Photosensitive Autocatalytic Activity
An acrylate and self-catalyzed technology, applied in non-polymer adhesive additives, non-polymer organic compound adhesives, adhesive additives, etc., can solve poor surface dryness, poor toughness of cured products, and flexible adhesive layer sex issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
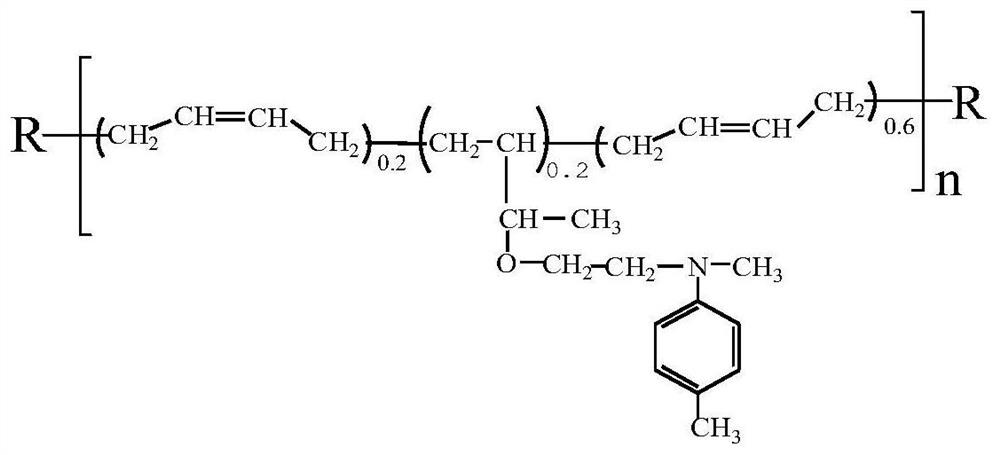
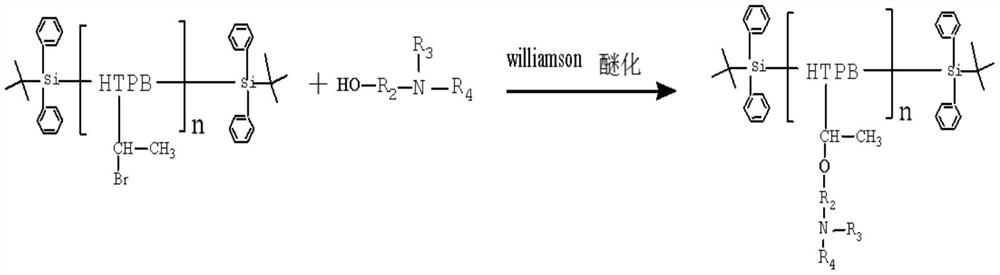
[0028] Take 430g of hydroxyl-terminated polybutadiene liquid rubber, add 30g of tert-butyldiphenylchlorosilane and 0.5g of diethyltetramethylimidazole under nitrogen protection, and react at 30°C for 4 hours to obtain silicon ether-protected rubber at both ends. the pretreatment;
[0029] Add the above-mentioned reactant to 22 g of HBr with a concentration of 40%, and react at 70° C. for 3 hours to obtain a halogenated hydrocarbon protected by silicon ether at both ends;
[0030] Add 55 g of N-methyl-N-hydroxyethyl-p-toluidine (CAS No. 2842-44-6) to the halogenated hydrocarbon protected with silicon ether at both ends prepared above, and react at 30°C under nitrogen protection for 3 hours;
[0031] Dissolve the product of the third step in tetrahydrofuran (THF) solvent, add catalyst tetraalkylammonium fluoride (TBAF) 0.26g, react at 25°C for 3-4h, and finally remove the solvent THF by rotary evaporation; after the above rotary evaporation The obtained product was vacuumized a...
Embodiment 2
[0033] Take 430g of hydroxyl-terminated polybutadiene liquid rubber, add 35g of tert-butyldiphenylchlorosilane and 1.5g of diethyltetramethylimidazole under nitrogen protection, and react at 40°C for 2 hours to obtain silicon ether-protected rubber at both ends. the pretreatment;
[0034] Add the above reactant to 20 g of HBr with a concentration of 40%, and react at 70°C for 3 hours to obtain a halogenated hydrocarbon protected by silicon ether at both ends;
[0035] Add 70 g of N-methyl-N-hydroxyethyl-p-toluidine (CAS No. 2842-44-6) to the halogenated hydrocarbon protected with silicon ether at both ends prepared above, and react at 30°C under nitrogen protection for 3 hours;
[0036] Dissolve the product of the third step in tetrahydrofuran (THF) solvent, add catalyst tetraalkylammonium fluoride (TBAF) 0.50g, react at 25°C for 3-4h, and finally remove the solvent THF by rotary evaporation; The obtained product was vacuumized at 110°C for 3 hours to remove moisture and othe...
Embodiment 3
[0038] Take 430g of hydroxyl-terminated polybutadiene liquid rubber, add 30g of tert-butyldiphenylchlorosilane and 1.0g of diethyltetramethylimidazole under nitrogen protection, and react at 40°C for 4 hours to obtain silicon ether-protected rubber at both ends. the pretreatment;
[0039] Add the above-mentioned reactant to 26 g of 40% HBr, and react at 70°C for 3 hours to obtain a halogenated hydrocarbon protected by silicon ether at both ends;
[0040] Add 48 g of N-methyl-N-hydroxyethyl-p-toluidine (CAS No. 2842-44-6) to the halogenated hydrocarbon protected with silicon ether at both ends prepared above, and react at 30°C under nitrogen protection for 3 hours;
[0041]Dissolve the product of the third step in tetrahydrofuran (THF) solvent, add catalyst tetraalkylammonium fluoride (TBAF) 0.38g, react at 25°C for 3-4h, and finally remove the solvent THF by rotary evaporation; after the above rotary evaporation The obtained product was vacuumized at 113°C for 2.5 hours to re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| storage modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com