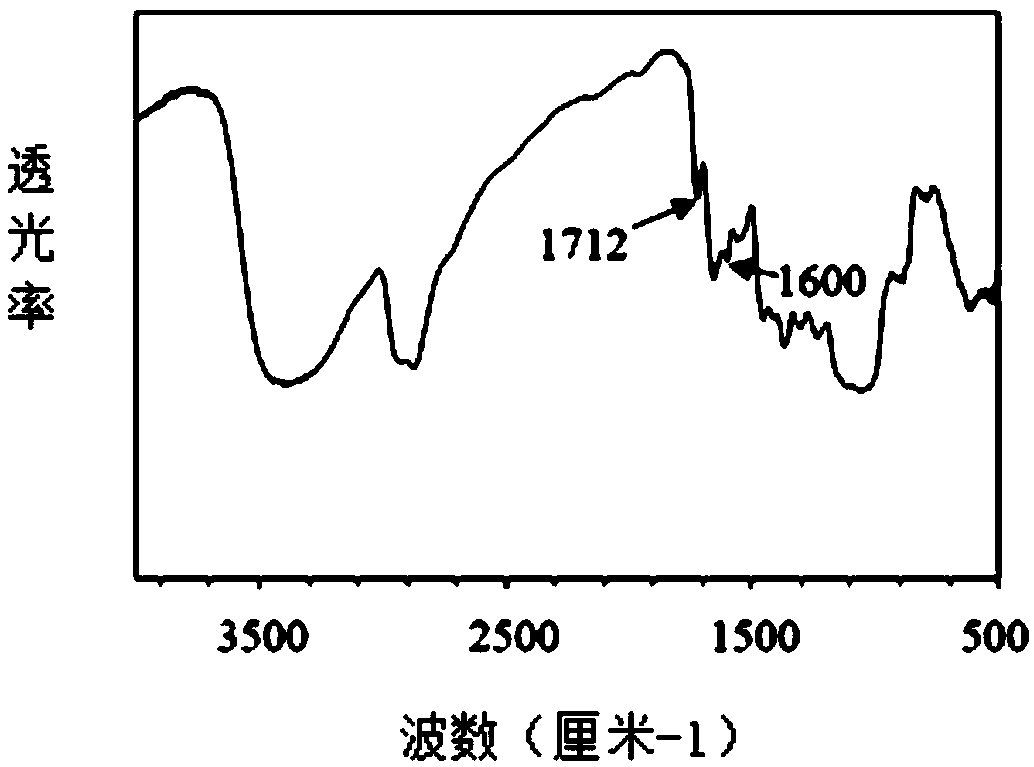
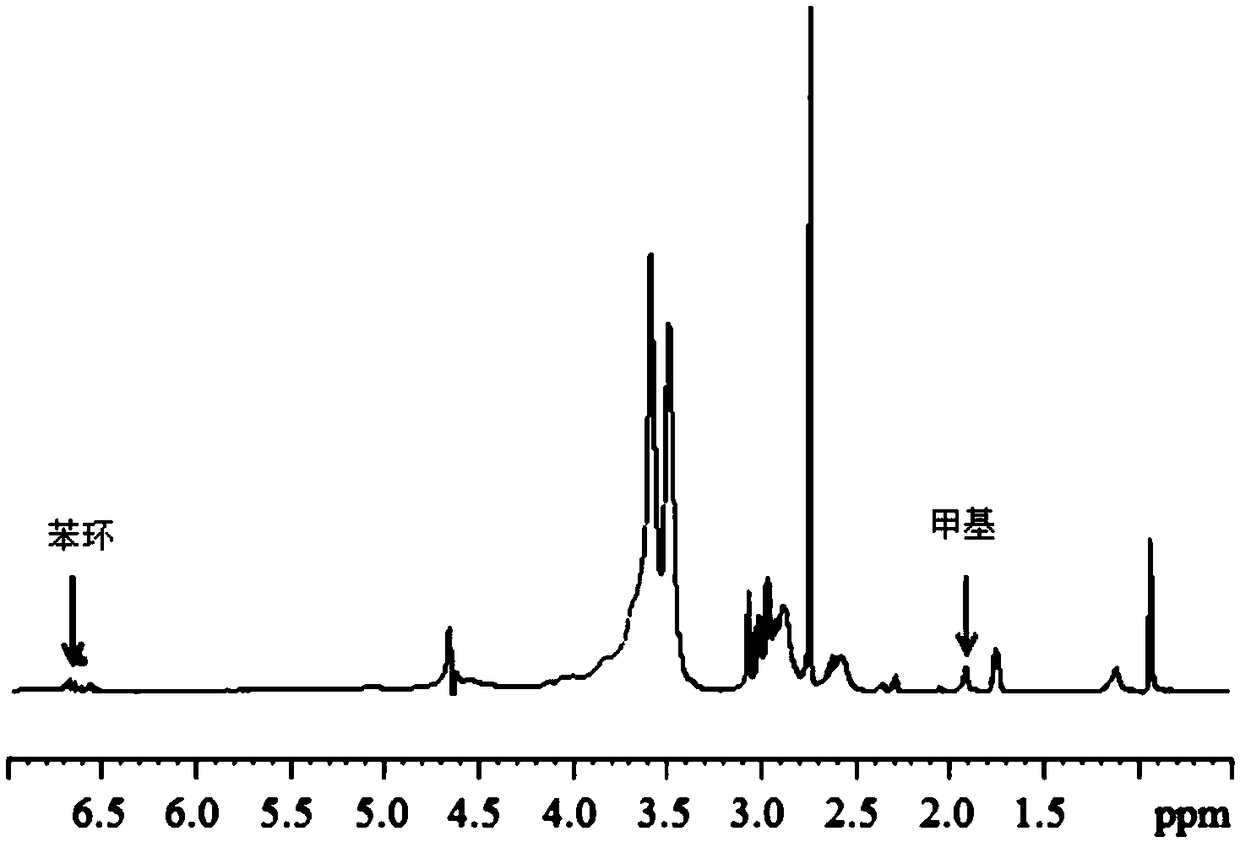
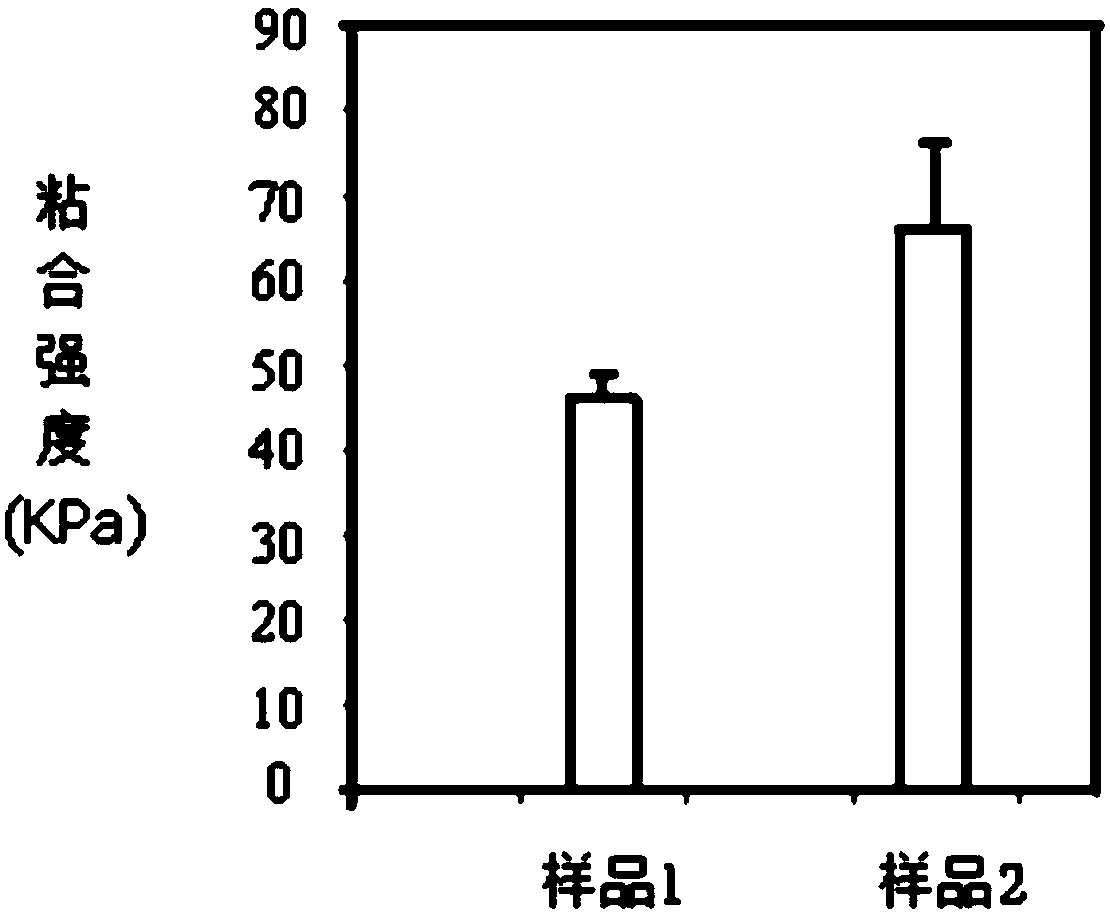
Chitosan-based hydrogel, and preparation method and application thereof
A technology of chitosan and hydrogel, which is applied in the field of medical materials, can solve problems such as limited applications, and achieve the effect of promoting wound healing, good biocompatibility, and excellent biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Hydrogel 1
[0028] Weigh 0.5 g of chitosan with a deacetylation degree of 90% and an apparent molecular weight of 400 kDa in a round-bottomed flask, add 50 mL of phosphate buffer solution with a pH of 6.5, and stir thoroughly to dissolve it completely. Weigh 0.43 g EDC and 0.17 g NHS and put them in a beaker, mix them with 25 mL of ethanol solution containing 0.13 g 3,4-dihydroxyphenylpropionic acid, add this mixture into the chitosan solution, and stir the reaction rapidly for 24 h, after the reaction, the mixture was first dialyzed in HCl solution with pH 5.0 for 2 days. Finally, the product is freeze-dried to obtain white spongy grafted phthalo-dihydroxy chitosan. After irradiation sterilization, the grafted o-phthalic dihydroxy chitosan was stirred and dissolved in a sterile phosphate buffer solution with a pH of 6.5 to a concentration of 13% by weight, and a concentration of 3.25 mg / mL period iodine was added. Sodium acid sterile aqueous solution, so ...
Embodiment 2
[0029] Example 2: Hydrogel 2
[0030] Weigh 0.5 g of hydroxyethyl chitosan and place it in a round bottom flask, add 50 mL of phosphate buffer solution with a pH of 6.5, stir well to dissolve it completely. Weigh 0.55 g EDC and 0.21 g NHS and place them in a beaker, mix them with 25 mL of ethanol solution containing 0.17 g 3,4-dihydroxyphenylpropionic acid, add this mixture to the hydroxyethyl chitosan solution, and quickly The reaction was stirred for 24 h. After the reaction, the mixture was dialyzed in HCl solution with pH 5.0 for 2 days. Finally, the product was freeze-dried to obtain white spongy grafted phthalic dihydroxy hydroxyethyl chitosan, wherein the grafting rate of phthalic dihydroxy groups was 14%. Stir and dissolve the grafted o-phthalic dihydroxy hydroxyethyl chitosan after irradiation sterilization in a sterile phosphate buffer solution with a pH of 6.5 to make its weight percent concentration 13%, and add a concentration of 3.25% under stirring conditions. ...
Embodiment 3
[0031] Example 3: Hydrogel 3
[0032] Weigh 0.5 g of hydroxypropyl chitosan and place it in a round bottom flask, add 50 mL of phosphate buffer solution with a pH of 6.5, stir well to dissolve it completely. Weigh 0.59 g of EDC and 0.22 g of NHS and mix with 25 mL of 3,4-dihydroxyphenylacetic acid ethanol solution containing 0.16 g, add this mixture into the hydroxypropyl chitosan solution, and stir rapidly for 24 h. After the end, the mixed solution was dialyzed, and then freeze-dried to obtain a white spongy grafted o-phthalic dihydroxy hydroxypropyl chitosan solid. After irradiation sterilization, the grafted o-phenylenedihydroxypropyl chitosan was stirred and dissolved in a sterile phosphate buffer solution with a pH of 6.5, so that the concentration by weight was 13%, and the added concentration was 3.25 mg / mL Sodium periodate sterile aqueous solution, make the molar ratio of the grafted o-phthalic dihydroxy group and sodium periodate be 1:0.25, mix well, constitute the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com